Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones
- PMID: 20668670
- PMCID: PMC2909897
- DOI: 10.1371/journal.pone.0011768
Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones
Abstract
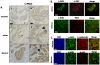
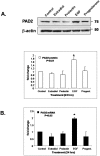
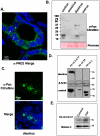
Peptidylarginine Deiminases (PADs) convert arginine residues on substrate proteins to citrulline. Previous reports have documented that PAD2 expression and activity varies across the estrous cycle in the rodent uterus and pituitary gland, however, the expression and function of PAD2 in mammary tissue has not been previously reported. To gain more insight into potential reproductive roles for PAD2, in this study we evaluated PAD2 expression and localization throughout the estrous cycle in canine mammary tissue and then identified possible PAD2 enzymatic _targets. Immunohistochemical and immunofluorescence analysis found PAD2 expression is low in anestrus, limited to a distinct, yet sparse, subset of epithelial cells within ductal alveoli during estrus/early diestrus, and encompasses the entire epithelium of the mammary duct in late diestrus. At the subcellular level, PAD2 is expressed in the cytoplasm, and to a lesser extent, the nucleus of these epithelial cells. Surprisingly, stimulation of canine mammary tumor cells (CMT25) shows that EGF, but not estrogen or progesterone, upregulates PAD2 transcription and translation suggesting EGF regulation of PAD2 and possibly citrullination in vivo. To identify potential PAD2 _targets, anti-pan citrulline western blots were performed and results showed that citrullination activity is limited to diestrus with histones appearing to represent major enzymatic _targets. Use of site-specific anti-citrullinated histone antibodies found that the N-terminus of histone H3, but not H4, appears to be the primary _target of PAD activity in mammary epithelium. This observation supports the hypothesis that PAD2 may play a regulatory role in the expression of lactation related genes via histone citrullination during diestrus.
Conflict of interest statement
Figures

Similar articles
-
GnRH Stimulates Peptidylarginine Deiminase Catalyzed Histone Citrullination in Gonadotrope Cells.Mol Endocrinol. 2016 Oct;30(10):1081-1091. doi: 10.1210/me.2016-1085. Epub 2016 Sep 7. Mol Endocrinol. 2016. PMID: 27603413 Free PMC article.
-
Comparative analysis of peptidylarginine deiminase-2 expression in canine, feline and human mammary tumours.J Comp Pathol. 2012 Aug-Oct;147(2-3):139-46. doi: 10.1016/j.jcpa.2012.01.021. Epub 2012 Apr 19. J Comp Pathol. 2012. PMID: 22520816
-
Potential role for PAD2 in gene regulation in breast cancer cells.PLoS One. 2012;7(7):e41242. doi: 10.1371/journal.pone.0041242. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22911765 Free PMC article.
-
Peptidylarginine deiminase enzymes and citrullinated proteins in female reproductive physiology and associated diseases†.Biol Reprod. 2022 Dec 10;107(6):1395-1410. doi: 10.1093/biolre/ioac173. Biol Reprod. 2022. PMID: 36087287 Free PMC article. Review.
-
Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration.Prion. 2013 Jan-Feb;7(1):42-6. doi: 10.4161/pri.22380. Epub 2012 Sep 28. Prion. 2013. PMID: 23022892 Free PMC article. Review.
Cited by
-
Symmetrically dimethylated histone H3R2 promotes global transcription during minor zygotic genome activation in mouse pronuclei.Sci Rep. 2021 May 12;11(1):10146. doi: 10.1038/s41598-021-89334-w. Sci Rep. 2021. PMID: 33980975 Free PMC article.
-
Crossing epigenetic frontiers: the intersection of novel histone modifications and diseases.Signal Transduct _target Ther. 2024 Sep 16;9(1):232. doi: 10.1038/s41392-024-01918-w. Signal Transduct _target Ther. 2024. PMID: 39278916 Free PMC article. Review.
-
A Pilot Study on Peptidylarginine Deiminases and Protein Deimination in Animal Cancers across Vertebrate Species.Int J Mol Sci. 2022 Aug 4;23(15):8697. doi: 10.3390/ijms23158697. Int J Mol Sci. 2022. PMID: 35955829 Free PMC article.
-
A FluoPol-ABPP PAD2 high-throughput screen identifies the first calcium site inhibitor _targeting the PADs.ACS Chem Biol. 2014 Apr 18;9(4):913-21. doi: 10.1021/cb400841k. Epub 2014 Jan 27. ACS Chem Biol. 2014. PMID: 24467619 Free PMC article.
-
Mechanistic studies of protein arginine deiminase 2: evidence for a substrate-assisted mechanism.Biochemistry. 2014 Jul 15;53(27):4426-33. doi: 10.1021/bi500554b. Epub 2014 Jul 3. Biochemistry. 2014. PMID: 24989433 Free PMC article.
References
-
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. - PubMed
-
- Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–1677. - PubMed
-
- Chavanas S, Mechin MC, Takahara H, Kawada A, Nachat R, et al. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6. Gene. 2004;330:19–27. - PubMed
-
- Vossenaar ER, Nijenhuis S, Helsen MM, van der Heijden A, Senshu T, et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489–2500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

