Different roles of DosS and DosT in the hypoxic adaptation of Mycobacteria
- PMID: 20675480
- PMCID: PMC2944544
- DOI: 10.1128/JB.00550-10
Different roles of DosS and DosT in the hypoxic adaptation of Mycobacteria
Abstract
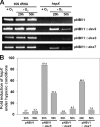
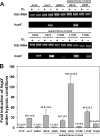
The DosS (DevS) and DosT histidine kinases form a two-component system together with the DosR (DevR) response regulator in Mycobacterium tuberculosis. DosS and DosT, which have high sequence similarity to each other over the length of their amino acid sequences, contain two GAF domains (GAF-A and GAF-B) in their N-terminal sensory domains. Complementation tests in conjunction with phylogenetic analysis showed that DevS of Mycobacterium smegmatis is more closely related to DosT than DosS. We also demonstrated in vivo that DosS and DosT of M. tuberculosis play a differential role in hypoxic adaptation. DosT responds to a decrease in oxygen tension more sensitively and strongly than DosS, which might be attributable to their different autooxidation rates. The different responsiveness of DosS and DosT to hypoxia is due to the difference in their GAF-A domains accommodating the hemes. Multiple alignment analysis of the GAF-A domains of mycobacterial DosS (DosT) homologs and subsequent site-directed mutagenesis revealed that just one substitution of E87, D90, H97, L118, or T169 of DosS with the corresponding residue of DosT is sufficient to convert DosS to DosT with regard to the responsiveness to changes in oxygen tension.
Figures




Similar articles
-
DosS responds to a reduced electron transport system to induce the Mycobacterium tuberculosis DosR regulon.J Bacteriol. 2010 Dec;192(24):6447-55. doi: 10.1128/JB.00978-10. Epub 2010 Oct 15. J Bacteriol. 2010. PMID: 20952575 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Mapping essential domains of Mycobacterium smegmatis WhmD: insights into WhiB structure and function.J Bacteriol. 2006 Oct;188(19):6966-76. doi: 10.1128/JB.00384-06. J Bacteriol. 2006. PMID: 16980499 Free PMC article.
-
"I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
-
DosR's multifaceted role on Mycobacterium bovis BCG revealed through multi-omics.Front Cell Infect Microbiol. 2023 Nov 21;13:1292864. doi: 10.3389/fcimb.2023.1292864. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38076461 Free PMC article.
-
The Partner Switching System of the SigF Sigma Factor in Mycobacterium smegmatis and Induction of the SigF Regulon Under Respiration-Inhibitory Conditions.Front Microbiol. 2020 Nov 11;11:588487. doi: 10.3389/fmicb.2020.588487. eCollection 2020. Front Microbiol. 2020. PMID: 33304334 Free PMC article.
-
Dual control of RegX3 transcriptional activity by SenX3 and PknB.J Biol Chem. 2019 Jul 12;294(28):11023-11034. doi: 10.1074/jbc.RA119.008232. Epub 2019 Jun 3. J Biol Chem. 2019. PMID: 31160336 Free PMC article.
-
Protein-protein interactions between histidine kinases and response regulators of Mycobacterium tuberculosis H37Rv.J Microbiol. 2012 Apr;50(2):270-7. doi: 10.1007/s12275-012-2050-4. Epub 2012 Apr 27. J Microbiol. 2012. PMID: 22538656
-
Regulation of the ahpC gene encoding alkyl hydroperoxide reductase in Mycobacterium smegmatis.PLoS One. 2014 Nov 3;9(11):e111680. doi: 10.1371/journal.pone.0111680. eCollection 2014. PLoS One. 2014. PMID: 25365321 Free PMC article.
References
-
- Brantley, R. E., S. J. Smerdon, A. J. Wilkinson, E. W. Singleton, and J. S. Olson. 1993. The mechanism of autooxidation of myoglobin. J. Biol. Chem. 268:6995-7010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

