A combined proteomics and metabolomics profiling of gastric cardia cancer reveals characteristic dysregulations in glucose metabolism
- PMID: 20699381
- PMCID: PMC3101851
- DOI: 10.1074/mcp.M110.000661
A combined proteomics and metabolomics profiling of gastric cardia cancer reveals characteristic dysregulations in glucose metabolism
Abstract
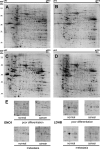
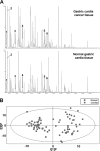
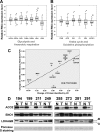
Gastric cardia cancer (GCC), which occurs at the gastric-esophageal boundary, is one of the most malignant tumors. Despite its high mortality and morbidity, the molecular mechanism of initiation and progression of this disease is largely unknown. In this study, using proteomics and metabolomics approaches, we found that the level of several enzymes and their related metabolic intermediates involved in glucose metabolism were deregulated in GCC. Among these enzymes, two subunits controlling pyruvic acid efflux, lactate dehydrogenase A (LDHA) and pyruvate dehydrogenase B (PDHB), were further analyzed in vitro. Either down-regulation of LDH subunit LDHA or overexpression of PDH subunit PDHB could force pyruvic acid into the Krebs cycle rather than the glycolysis process in AGS gastric cancer cells, which inhibited cell growth and cell migration. Our results reflect an important glucose metabolic signature, especially the dysregulation of pyruvic acid efflux in the development of GCC. Forced transition from glycolysis to the Krebs cycle had an inhibitory effect on GCC progression, providing potential therapeutic _targets for this disease.
Figures
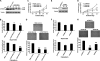
Similar articles
-
Stable shRNA Silencing of Lactate Dehydrogenase A (LDHA) in Human MDA-MB-231 Breast Cancer Cells Fails to Alter Lactic Acid Production, Glycolytic Activity, ATP or Survival.Anticancer Res. 2017 Mar;37(3):1205-1212. doi: 10.21873/anticanres.11435. Anticancer Res. 2017. PMID: 28314283 Free PMC article.
-
FOXM1-LDHA signaling promoted gastric cancer glycolytic phenotype and progression.Int J Clin Exp Pathol. 2015 Jun 1;8(6):6756-63. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26261559 Free PMC article.
-
TOP1MT deficiency promotes GC invasion and migration via the enhancements of LDHA expression and aerobic glycolysis.Endocr Relat Cancer. 2017 Nov;24(11):565-578. doi: 10.1530/ERC-17-0058. Epub 2017 Sep 5. Endocr Relat Cancer. 2017. PMID: 28874393 Free PMC article.
-
Enzymes involved in l-lactate metabolism in humans.Mitochondrion. 2013 Nov;13(6):615-29. doi: 10.1016/j.mito.2013.08.011. Epub 2013 Sep 9. Mitochondrion. 2013. PMID: 24029012 Review.
-
Glucose metabolism in gastric cancer: The cutting-edge.World J Gastroenterol. 2016 Feb 14;22(6):2046-59. doi: 10.3748/wjg.v22.i6.2046. World J Gastroenterol. 2016. PMID: 26877609 Free PMC article. Review.
Cited by
-
Glycylglycine plays critical roles in the proliferation of spermatogonial stem cells.Mol Med Rep. 2019 Oct;20(4):3802-3810. doi: 10.3892/mmr.2019.10609. Epub 2019 Aug 23. Mol Med Rep. 2019. PMID: 31485625 Free PMC article.
-
PCK1 Regulates Glycolysis and Tumor Progression in Clear Cell Renal Cell Carcinoma Through LDHA.Onco _targets Ther. 2020 Mar 30;13:2613-2627. doi: 10.2147/OTT.S241717. eCollection 2020. Onco _targets Ther. 2020. PMID: 32280238 Free PMC article.
-
Potential role of metabolomics in diagnosis and surveillance of gastric cancer.World J Gastroenterol. 2014 Sep 28;20(36):12874-82. doi: 10.3748/wjg.v20.i36.12874. World J Gastroenterol. 2014. PMID: 25278684 Free PMC article. Review.
-
A novel long non-coding RNA ENST00000480739 suppresses tumour cell invasion by regulating OS-9 and HIF-1α in pancreatic ductal adenocarcinoma.Br J Cancer. 2014 Nov 25;111(11):2131-41. doi: 10.1038/bjc.2014.520. Epub 2014 Oct 14. Br J Cancer. 2014. PMID: 25314054 Free PMC article.
-
Metabolomics reveals metabolic changes in male reproductive cells exposed to thirdhand smoke.Sci Rep. 2015 Oct 22;5:15512. doi: 10.1038/srep15512. Sci Rep. 2015. PMID: 26489853 Free PMC article.
References
-
- He Q. Y., Cheung Y. H., Leung S. Y., Yuen S. T., Chu K. M., Chiu J. F. (2004) Diverse proteomic alterations in gastric adenocarcinoma. Proteomics 4, 3276–3287 - PubMed
-
- Botterweck A. A., Schouten L. J., Volovics A., Dorant E., van Den Brandt P. A. (2000) Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int. J. Epidemiol. 29, 645–654 - PubMed
-
- Bareiss D., Stabenow R., Müller R., Eisinger B., Stegmaier C., Däubler P., Zeitz M., Scherübl H. (2002) Current epidemiology of carcinoma of the esophagus and cardia in Germany. Dtsch. Med. Wochenschr. 127, 1367–1374 - PubMed
-
- Wijnhoven B. P., Louwman M. W., Tilanus H. W., Coebergh J. W. (2002) Increased incidence of adenocarcinomas at the gastrooesophageal junction in Dutch males since the 1990s. Eur. J. Gastroenterol. Hepatol. 14, 115–122 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

