Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling
- PMID: 20699479
- PMCID: PMC2931533
- DOI: 10.1242/dmm.006031
Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling
Abstract
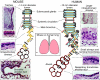
The small airways of the human lung undergo pathological changes in pulmonary disorders, such as chronic obstructive pulmonary disease (COPD), asthma, bronchiolitis obliterans and cystic fibrosis. These clinical problems impose huge personal and societal healthcare burdens. The changes, termed 'pathological airway remodeling', affect the epithelium, the underlying mesenchyme and the reciprocal trophic interactions that occur between these tissues. Most of the normal human airway is lined by a pseudostratified epithelium of ciliated cells, secretory cells and 6-30% basal cells, the proportion of which varies along the proximal-distal axis. Epithelial abnormalities range from hypoplasia (failure to differentiate) to basal- and goblet-cell hyperplasia, squamous- and goblet-cell metaplasia, dysplasia and malignant transformation. Mesenchymal alterations include thickening of the basal lamina, smooth muscle hyperplasia, fibrosis and inflammatory cell accumulation. Paradoxically, given the prevalence and importance of airway remodeling in lung disease, its etiology is poorly understood. This is due, in part, to a lack of basic knowledge of the mechanisms that regulate the differentiation, maintenance and repair of the airway epithelium. Specifically, little is known about the proliferation and differentiation of basal cells, a multipotent stem cell population of the pseudostratified airway epithelium. This Perspective summarizes what we know, and what we need to know, about airway basal cells to evaluate their contributions to normal and abnormal airway remodeling. We contend that exploiting well-described model systems using both human airway epithelial cells and the pseudostratified epithelium of the genetically tractable mouse trachea will enable crucial discoveries regarding the pathogenesis of airway disease.
Figures


Similar articles
-
Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells.Ann Am Thorac Soc. 2014 Dec;11 Suppl 5(Suppl 5):S252-8. doi: 10.1513/AnnalsATS.201402-049AW. Ann Am Thorac Soc. 2014. PMID: 25525728 Free PMC article. Review.
-
Exaggerated BMP4 signalling alters human airway basal progenitor cell differentiation to cigarette smoking-related phenotypes.Eur Respir J. 2019 May 18;53(5):1702553. doi: 10.1183/13993003.02553-2017. Print 2019 May. Eur Respir J. 2019. PMID: 30705127 Free PMC article.
-
Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge.Am J Respir Cell Mol Biol. 2013 Mar;48(3):364-73. doi: 10.1165/rcmb.2012-0146OC. Epub 2012 Dec 13. Am J Respir Cell Mol Biol. 2013. PMID: 23239495 Free PMC article.
-
Increased expression of TROP2 in airway basal cells potentially contributes to airway remodeling in chronic obstructive pulmonary disease.Respir Res. 2016 Nov 25;17(1):159. doi: 10.1186/s12931-016-0463-z. Respir Res. 2016. PMID: 27887617 Free PMC article.
-
The non-human primate as a model for studying COPD and asthma.Pulm Pharmacol Ther. 2008 Oct;21(5):755-66. doi: 10.1016/j.pupt.2008.01.008. Epub 2008 Feb 1. Pulm Pharmacol Ther. 2008. PMID: 18339566 Review.
Cited by
-
Comprehensive evaluation of poly(I:C) induced inflammatory response in an airway epithelial model.Physiol Rep. 2015 Apr;3(4):e12334. doi: 10.14814/phy2.12334. Physiol Rep. 2015. PMID: 25847914 Free PMC article.
-
Macrophages Inhibit Ciliary Protein Levels by Secreting BMP-2 Leading to Airway Epithelial Remodeling Under Cigarette Smoke Exposure.Front Mol Biosci. 2021 Apr 26;8:663987. doi: 10.3389/fmolb.2021.663987. eCollection 2021. Front Mol Biosci. 2021. PMID: 33981724 Free PMC article.
-
Multi-apical polarity of alveolar stem cells and their dynamics during lung development and regeneration.iScience. 2022 Sep 12;25(10):105114. doi: 10.1016/j.isci.2022.105114. eCollection 2022 Oct 21. iScience. 2022. PMID: 36185377 Free PMC article.
-
In vivo correction of cystic fibrosis mediated by PNA nanoparticles.Sci Adv. 2022 Oct 7;8(40):eabo0522. doi: 10.1126/sciadv.abo0522. Epub 2022 Oct 5. Sci Adv. 2022. PMID: 36197984 Free PMC article.
-
De_targeting Lentiviral-Mediated CFTR Expression in Airway Basal Cells Using miR-106b.Genes (Basel). 2020 Oct 6;11(10):1169. doi: 10.3390/genes11101169. Genes (Basel). 2020. PMID: 33036232 Free PMC article.
References
-
- Avril-Delplanque A, Casal I, Castillon N, Hinnrasky J, Puchelle E, Peault B. (2005). Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells 23, 992–1001 - PubMed
-
- Banerjee AK. (2009). Preinvasive lesions of the bronchus. J Thorac Oncol. 4, 545–551 - PubMed
-
- Batlle E. (2008). A new identity for the elusive intestinal stem cell. Nat Genet. 40, 818–819 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

