Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy
- PMID: 20798685
- PMCID: PMC3131882
- DOI: 10.1038/cdd.2010.104
Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy
Abstract
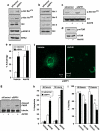
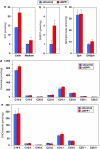
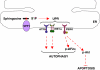
The sphingolipid metabolites ceramide and sphingosine-1-phosphate (S1P) have recently been implicated in autophagy. In this study, we report that depletion of sphingosine-1-phosphate phosphohydrolase-1 (SPP1), an endoplasmic reticulum (ER)-resident enzyme that specifically dephosphorylates S1P, induced autophagy. Although the mammalian _target of rapamycin and class III phosphoinositide 3-kinase/Beclin-1 pathways were not involved and this autophagy was p53 independent, C/EBP homologous protein, BiP, and phospho-eucaryotic translation initiation factor-2α, and cleavage of procaspases 2 and 4, downstream _targets of ER stress, were increased after SPP1 depletion. Autophagy was suppressed by depletion of protein kinase regulated by RNA-like ER kinase (PERK), inositol-requiring transmembrane kinase/endonuclease-1α, or activating transcription factor 6, three sensors of the unfolded protein response (UPR) to ER stress. Autophagy triggered by downregulation of SPP1 did not lead to apoptosis but rather stimulated, in a PERK dependent manner, the survival signal Akt, whose inhibition then sensitized cells to apoptosis. Although depletion of SPP1 increased intracellular levels of S1P and its secretion, activation of cell surface S1P receptors did not induce autophagy. Nevertheless, increases in intracellular pools of S1P, but not dihydro-S1P, induced autophagy and ER stress. Thus, SPP1, by regulating intracellular S1P homeostasis, can control the UPR and ER stress-induced autophagy.
Figures

Similar articles
-
Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated autophagy-related gene 5 (Atg5) cleavage.J Biol Chem. 2011 Dec 30;286(52):44380-90. doi: 10.1074/jbc.M111.257519. Epub 2011 Nov 3. J Biol Chem. 2011. PMID: 22052905 Free PMC article.
-
Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-golgi trafficking of ceramide.Mol Cell Biol. 2006 Jul;26(13):5055-69. doi: 10.1128/MCB.02107-05. Mol Cell Biol. 2006. PMID: 16782891 Free PMC article.
-
Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate.J Lipid Res. 2010 Sep;51(9):2546-59. doi: 10.1194/jlr.M004374. Epub 2010 Apr 12. J Lipid Res. 2010. PMID: 20386061 Free PMC article.
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
Sphingosine-1-phosphate and lipid phosphohydrolases.Biochim Biophys Acta. 2002 May 23;1582(1-3):8-17. doi: 10.1016/s1388-1981(02)00132-4. Biochim Biophys Acta. 2002. PMID: 12069805 Review.
Cited by
-
Fanning the Flames of Endoplasmic Reticulum (ER) Stress: Can Sphingolipid Metabolism Be _targeted to Enhance ER Stress-Associated Immunogenic Cell Death in Cancer?Mol Pharmacol. 2024 Feb 15;105(3):155-165. doi: 10.1124/molpharm.123.000786. Mol Pharmacol. 2024. PMID: 38164594 Free PMC article. Review.
-
Dysregulation of ceramide metabolism causes phytoceramide-dependent induction of the unfolded protein response.Mol Biol Cell. 2024 Sep 1;35(9):ar117. doi: 10.1091/mbc.E24-03-0121. Epub 2024 Jul 18. Mol Biol Cell. 2024. PMID: 39024283 Free PMC article.
-
Sphingosine 1 phosphate receptor-1 (S1PR1) signaling protects cardiac function by inhibiting cardiomyocyte autophagy.J Geriatr Cardiol. 2018 May;15(5):334-345. doi: 10.11909/j.issn.1671-5411.2018.05.003. J Geriatr Cardiol. 2018. PMID: 30083186 Free PMC article.
-
Sphingolipid Metabolism: A New Therapeutic Opportunity for Brain Degenerative Disorders.Front Neurosci. 2018 Apr 17;12:249. doi: 10.3389/fnins.2018.00249. eCollection 2018. Front Neurosci. 2018. PMID: 29719499 Free PMC article. Review.
-
Runx1 Orchestrates Sphingolipid Metabolism and Glucocorticoid Resistance in Lymphomagenesis.J Cell Biochem. 2017 Jun;118(6):1432-1441. doi: 10.1002/jcb.25802. Epub 2017 Jan 10. J Cell Biochem. 2017. PMID: 27869314 Free PMC article.
References
-
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. - PubMed
-
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. - PubMed
-
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. - PubMed
-
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous


