Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo
- PMID: 20889562
- PMCID: PMC2966812
- DOI: 10.2353/ajpath.2010.090941
Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo
Abstract
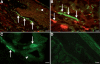
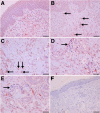
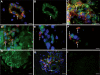
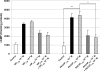
Pituitary adenylate cyclase-activating peptide (PACAP) is an important neuropeptide and immunomodulator in various tissues. Although this peptide and its receptors (ie, VPAC1R, VPAC2R, and PAC1R) are expressed in human skin, their biological roles are unknown. Therefore, we tested whether PACAP regulates vascular responses in human skin in vivo. When injected intravenously, PACAP induced a significant, concentration-dependent vascular response (ie, flush, erythema, edema) and mediated a significant and concentration-dependent increase in intrarectal body temperature that peaked at 2.7°C. Topical application of PACAP induced marked concentration-dependent edema. Immunohistochemistry revealed a close association of PACAP-immunoreactive nerve fibers with mast cells and dermal blood vessels. VPAC1R was expressed by dermal endothelial cells, CD4+ and CD8+ T cells, mast cells, and keratinocytes, whereas VPAC2R was expressed only in keratinocytes. VPAC1R protein and mRNA were also detected in human dermal microvascular endothelial cells. The PACAP-induced change in cAMP production in these cells demonstrated VPAC1R to be functional. PACAP treatment of organ-cultured human skin strongly increased the number of CD31+ vessel cross-sections. Taken together, these results suggest that PACAP directly induces vascular responses that may be associated with neurogenic inflammation, indicating for the first time that PACAP may be a crucial vascular regulator in human skin in vivo. Antagonists to PACAP function may be beneficial for the treatment of inflammatory skin diseases with a neurogenic component.
Figures



Similar articles
-
Expression localisation and functional activity of pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal polypeptide and their receptors in mouse ovary.Reproduction. 2007 Aug;134(2):281-92. doi: 10.1530/REP-07-0051. Reproduction. 2007. PMID: 17660238
-
PAC1 receptors mediate pituitary adenylate cyclase-activating polypeptide- and progesterone-facilitated receptivity in female rats.Mol Endocrinol. 2005 Nov;19(11):2798-811. doi: 10.1210/me.2004-0387. Epub 2005 Jun 23. Mol Endocrinol. 2005. PMID: 15976009
-
Pituitary adenylate cyclase-activating polypeptide promotes eccrine gland sweat secretion.Br J Dermatol. 2017 Feb;176(2):413-422. doi: 10.1111/bjd.14885. Epub 2017 Jan 24. Br J Dermatol. 2017. PMID: 27453364
-
_targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors.Int J Mol Sci. 2022 Jul 22;23(15):8069. doi: 10.3390/ijms23158069. Int J Mol Sci. 2022. PMID: 35897648 Free PMC article. Review.
-
PACAP and its receptors in cranial arteries and mast cells.J Headache Pain. 2018 Feb 20;19(1):16. doi: 10.1186/s10194-017-0822-2. J Headache Pain. 2018. PMID: 29460121 Free PMC article. Review.
Cited by
-
Pituitary adenylate cyclase-activating polypeptide (PACAP) signalling enhances osteogenesis in UMR-106 cell line.J Mol Neurosci. 2014 Nov;54(3):555-73. doi: 10.1007/s12031-014-0389-1. Epub 2014 Aug 12. J Mol Neurosci. 2014. PMID: 25112418
-
Clinical characteristics of combined rosacea and migraine.Front Med (Lausanne). 2022 Oct 20;9:1026447. doi: 10.3389/fmed.2022.1026447. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36341245 Free PMC article.
-
Pruritus, Allergy and Autoimmunity: Paving the Way for an Integrated Understanding of Psychodermatological Diseases?Front Allergy. 2021 Sep 17;2:688999. doi: 10.3389/falgy.2021.688999. eCollection 2021. Front Allergy. 2021. PMID: 35387041 Free PMC article.
-
Skin neurogenic inflammation.Semin Immunopathol. 2018 May;40(3):249-259. doi: 10.1007/s00281-018-0675-z. Epub 2018 Apr 30. Semin Immunopathol. 2018. PMID: 29713744 Free PMC article. Review.
-
Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models.Mol Vis. 2021 May 13;27:323-353. eCollection 2021. Mol Vis. 2021. PMID: 34035646 Free PMC article. Review.
References
-
- Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488. - PubMed
-
- Cevikbas F, Steinhoff A, Homey B, Steinhoff M. Neuroimmune interactions in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2007;7:365–373. - PubMed
-
- Blais C, Jr, Rouleau JL, Brown NJ, Lepage Y, Spence D, Munoz C, Friborg J, Geadah D, Gervais N, Adam A. Serum metabolism of bradykinin and des-Arg9-bradykinin in patients with angiotensin-converting enzyme inhibitor-associated angioedema. Immunopharmacology. 1999;43:293–302. - PubMed
-
- Nussberger J, Cugno M, Cicardi M, Agostoni A. Local bradykinin generation in hereditary angioedema. J Allergy Clin Immunol. 1999;104:1321–1322. - PubMed
-
- Doutre M. Physiopathology of urticaria. Eur J Dermatol. 1999;9:601–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

