Histone H1 poly[ADP]-ribosylation regulates the chromatin alterations required for learning consolidation
- PMID: 20926656
- PMCID: PMC6634736
- DOI: 10.1523/JNEUROSCI.3010-10.2010
Histone H1 poly[ADP]-ribosylation regulates the chromatin alterations required for learning consolidation
Abstract
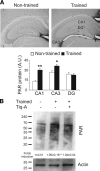
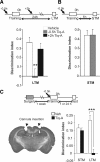
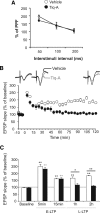
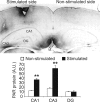
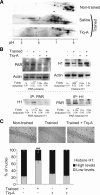
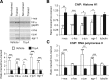
Memory formation requires changes in gene expression, which are regulated by the activation of transcription factors and by changes in epigenetic factors. Poly[ADP]-ribosylation of nuclear proteins has been postulated as a chromatin modification involved in memory consolidation, although the mechanisms involved are not well characterized. Here we demonstrate that poly[ADP]-ribose polymerase 1 (PARP-1) activity and the poly[ADP]-ribosylation of proteins over a specific time course is required for the changes in synaptic plasticity related to memory stabilization in mice. At the molecular level, histone H1 poly[ADP]-ribosylation was evident in the hippocampus after the acquisition period, and it was selectively released in a PARP-1-dependent manner at the promoters of cAMP response element-binding protein and nuclear factor-κB dependent genes associated with learning and memory. These findings suggest that histone H1 poly[ADP]-ribosylation, and its loss at specific loci, is an epigenetic mechanism involved in the reprogramming of neuronal gene expression required for memory consolidation.
Figures
Similar articles
-
Poly(ADP-ribosylation) regulates chromatin organization through histone H3 modification and DNA methylation of the first cell cycle of mouse embryos.Biochem Biophys Res Commun. 2013 Apr 26;434(1):15-21. doi: 10.1016/j.bbrc.2013.03.074. Epub 2013 Mar 30. Biochem Biophys Res Commun. 2013. PMID: 23548571
-
Alterations in the polynucleosomal structure of chromatin by poly ADP-ribosylation of nuclear proteins.Princess Takamatsu Symp. 1982;12:189-204. Princess Takamatsu Symp. 1982. PMID: 6300019
-
Relation between carcinogenesis, chromatin structure and poly(ADP-ribosylation) (review).Anticancer Res. 1991 Mar-Apr;11(2):489-527. Anticancer Res. 1991. PMID: 1905900 Review.
-
Poly(ADP-ribosyl)ation is required to modulate chromatin changes at c-MYC promoter during emergence from quiescence.PLoS One. 2014 Jul 21;9(7):e102575. doi: 10.1371/journal.pone.0102575. eCollection 2014. PLoS One. 2014. PMID: 25047032 Free PMC article.
-
Histone shuttling by poly ADP-ribosylation.Mol Cell Biochem. 1994 Sep;138(1-2):53-9. doi: 10.1007/BF00928443. Mol Cell Biochem. 1994. PMID: 7898476 Review.
Cited by
-
Evidence for Decreased Nucleolar PARP-1 as an Early Marker of Cognitive Impairment.Neural Plast. 2019 Nov 19;2019:4383258. doi: 10.1155/2019/4383258. eCollection 2019. Neural Plast. 2019. PMID: 31827497 Free PMC article.
-
PARP-1 is required for retrieval of cocaine-associated memory by binding to the promoter of a novel gene encoding a putative transposase inhibitor.Mol Psychiatry. 2017 Apr;22(4):570-579. doi: 10.1038/mp.2016.119. Epub 2016 Sep 6. Mol Psychiatry. 2017. PMID: 27595592
-
A Long-Lasting PARP1-Activation Mediates Signal-Induced Gene Expression.Cells. 2022 May 7;11(9):1576. doi: 10.3390/cells11091576. Cells. 2022. PMID: 35563882 Free PMC article. Review.
-
PARP1-dependent eviction of the linker histone H1 mediates immediate early gene expression during neuronal activation.J Cell Biol. 2018 Feb 5;217(2):473-481. doi: 10.1083/jcb.201703141. Epub 2017 Dec 28. J Cell Biol. 2018. PMID: 29284668 Free PMC article.
-
PARP1: Liaison of Chromatin Remodeling and Transcription.Cancers (Basel). 2022 Aug 27;14(17):4162. doi: 10.3390/cancers14174162. Cancers (Basel). 2022. PMID: 36077699 Free PMC article. Review.
References
-
- Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Rev. 1998;26:360–378. - PubMed
-
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. - PubMed
-
- Aguilar-Quesada R, Muñoz-Gámez JA, Martín-Oliva D, Peralta-Leal A, Quiles-Pérez R, Rodríguez-Vargas JM, Ruiz de Almodóvar M, Conde C, Ruiz-Extremera A, Oliver FJ. Modulation of transcription by PARP-1: consequences in carcinogenesis and inflammation. Curr Med Chem. 2007;14:1179–1187. - PubMed
-
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
