Epigenetic regulation of CpG promoter methylation in invasive prostate cancer cells
- PMID: 20929579
- PMCID: PMC2958982
- DOI: 10.1186/1476-4598-9-267
Epigenetic regulation of CpG promoter methylation in invasive prostate cancer cells
Abstract
Background: Recently, much attention has been focused on gaining a better understanding of the different populations of cells within a tumor and their contribution to cancer progression. One of the most commonly used methods to isolate a more aggressive sub-population of cells utilizes cell sorting based on expression of certain cell adhesion molecules. A recently established method we developed is to isolate these more aggressive cells based on their properties of increased invasive ability. These more invasive cells have been previously characterized as tumor initiating cells (TICs) that have a stem-like genomic signature and express a number of stem cell genes including Oct3/4 and Nanog and are more tumorigenic compared to their 'non-invasive' counterpart. They also have a profile reminiscent of cells undergoing a classic pattern of epithelial to mesenchymal transition or EMT. Using this model of invasion, we sought to investigate which genes are under epigenetic control in this rare population of cells. Epigenetic modifications, specifically DNA methylation, are key events regulating the process of normal human development. To determine the specific methylation pattern in these invasive prostate cells, and if any developmental genes were being differentially regulated, we analyzed differences in global CpG promoter methylation.
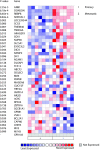
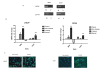
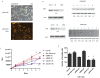
Results: Differentially methylated genes were determined and select genes were chosen for additional analyses. The non-receptor tyrosine kinase BMX and transcription factor SOX1 were found to play a significant role in invasion. Ingenuity pathway analysis revealed the methylated gene list frequently displayed genes from the IL-6/STAT3 pathway. Cells which have decreased levels of the _targets BMX and SOX1 also display loss of STAT3 activity. Finally, using Oncomine, it was determined that more aggressive metastatic prostate cancers in humans also have higher levels of both Stat3 and Sox1.
Conclusions: Using this method we can begin to understand which genes are epigenetically regulated in the invasive population compared to the bulk tumor cells. These aggressive sub-populations of cells may be linked to the cancer stem cell hypothesis, making their patterns of epigenetic regulation very attractive for biomarker analysis.
Figures

Similar articles
-
The ETS factor ESE3/EHF represses IL-6 preventing STAT3 activation and expansion of the prostate cancer stem-like compartment.Onco_target. 2016 Nov 22;7(47):76756-76768. doi: 10.18632/onco_target.12525. Onco_target. 2016. PMID: 27732936 Free PMC article.
-
Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling.J Hematol Oncol. 2015 Mar 11;8:23. doi: 10.1186/s13045-015-0119-3. J Hematol Oncol. 2015. PMID: 25879771 Free PMC article.
-
Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells.J Urol. 2010 May;183(5):2045-53. doi: 10.1016/j.juro.2009.12.092. Epub 2010 Mar 19. J Urol. 2010. PMID: 20303530 Free PMC article.
-
Aspects of the Epigenetic Regulation of EMT Related to Cancer Metastasis.Cells. 2021 Dec 6;10(12):3435. doi: 10.3390/cells10123435. Cells. 2021. PMID: 34943943 Free PMC article. Review.
-
The importance of DNA methylation in prostate cancer development.J Steroid Biochem Mol Biol. 2017 Feb;166:1-15. doi: 10.1016/j.jsbmb.2016.04.009. Epub 2016 Apr 24. J Steroid Biochem Mol Biol. 2017. PMID: 27117390 Review.
Cited by
-
Epigenetic reactivation of RANK in glioblastoma cells by curcumin: involvement of STAT3 inhibition.DNA Cell Biol. 2013 Jun;32(6):292-7. doi: 10.1089/dna.2013.2042. Epub 2013 Apr 27. DNA Cell Biol. 2013. PMID: 23621850 Free PMC article.
-
Generation of High-Throughput Three-Dimensional Tumor Spheroids for Drug Screening.J Vis Exp. 2018 Sep 5;(139):57476. doi: 10.3791/57476. J Vis Exp. 2018. PMID: 30247463 Free PMC article.
-
DNA methylation of PAX1 as a biomarker for oral squamous cell carcinoma.Clin Oral Investig. 2014 Apr;18(3):801-8. doi: 10.1007/s00784-013-1048-6. Epub 2013 Aug 2. Clin Oral Investig. 2014. PMID: 23907469
-
New therapy _targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells.J Mol Cell Biol. 2013 Feb;5(1):14-26. doi: 10.1093/jmcb/mjs042. Epub 2012 Jul 24. J Mol Cell Biol. 2013. PMID: 22831834 Free PMC article.
-
Genomic Analysis of Invasive Human Bone Marrow Derived Mesenchymal Stem Cells.J Bone Marrow Res. 2013 May 23;1:122. doi: 10.4172/2329-8820.1000122. J Bone Marrow Res. 2013. PMID: 24772452 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

