Steroid receptor coactivator (SRC) family: masters of systems biology
- PMID: 20956538
- PMCID: PMC2998129
- DOI: 10.1074/jbc.R110.193367
Steroid receptor coactivator (SRC) family: masters of systems biology
Abstract
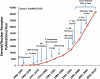
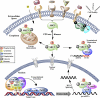
The three members of the p160 family of steroid receptor coactivators (SRC-1, SRC-2, and SRC-3) steer the functional output of numerous genetic programs and serve as pleiotropic rheostats for diverse physiological processes. Since their discovery ∼15 years ago, the extraordinary sum of examination of SRC function has shaped the foundation of our knowledge for the now 350+ coregulators that have been identified to date. In this perspective, we retrace our steps into the field of coregulators and provide a summary of selected seminal work that helped define the SRCs as masters of systems biology.
Figures
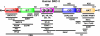
Similar articles
-
Steroid receptor coactivators: servants and masters for control of systems metabolism.Trends Endocrinol Metab. 2014 Jul;25(7):337-47. doi: 10.1016/j.tem.2014.05.004. Epub 2014 Jun 19. Trends Endocrinol Metab. 2014. PMID: 24953190 Free PMC article. Review.
-
An acetylation-enhanced interaction between transcription factor Sox2 and the steroid receptor coactivators facilitates Sox2 transcriptional activity and function.J Biol Chem. 2021 Dec;297(6):101389. doi: 10.1016/j.jbc.2021.101389. Epub 2021 Nov 8. J Biol Chem. 2021. PMID: 34762910 Free PMC article.
-
miR-137 _targets p160 Steroid Receptor Coactivators SRC1, SRC2, and SRC3 and Inhibits Cell Proliferation.Mol Endocrinol. 2015 Aug;29(8):1170-83. doi: 10.1210/me.2015-1080. Epub 2015 Jun 12. Mol Endocrinol. 2015. PMID: 26066330 Free PMC article.
-
Expression patterns of the steroid receptor coactivator family in human ovarian endometriosis.J Obstet Gynaecol Res. 2011 Oct;37(10):1269-76. doi: 10.1111/j.1447-0756.2010.01509.x. Epub 2011 Apr 26. J Obstet Gynaecol Res. 2011. PMID: 21518137
-
Steroid receptor coactivators present a unique opportunity for drug development in hormone-dependent cancers.Biochem Pharmacol. 2017 Sep 15;140:1-7. doi: 10.1016/j.bcp.2017.04.005. Epub 2017 Apr 6. Biochem Pharmacol. 2017. PMID: 28390937 Review.
Cited by
-
Modulation of testosterone-dependent male sexual behavior and the associated neuroplasticity.Gen Comp Endocrinol. 2013 Sep 1;190:24-33. doi: 10.1016/j.ygcen.2013.03.003. Epub 2013 Mar 20. Gen Comp Endocrinol. 2013. PMID: 23523709 Free PMC article.
-
Steroid receptor coactivator-3 inhibition generates breast cancer antitumor immune microenvironment.Breast Cancer Res. 2022 Oct 31;24(1):73. doi: 10.1186/s13058-022-01568-2. Breast Cancer Res. 2022. PMID: 36316775 Free PMC article.
-
"True" antiandrogens-selective non-ligand-binding pocket disruptors of androgen receptor-coactivator interactions: novel tools for prostate cancer.J Med Chem. 2012 Feb 23;55(4):1635-44. doi: 10.1021/jm201438f. Epub 2012 Feb 10. J Med Chem. 2012. PMID: 22280402 Free PMC article.
-
Regulation of HER2 oncogene transcription by a multifunctional coactivator/corepressor complex.Mol Endocrinol. 2014 Jun;28(6):846-59. doi: 10.1210/me.2013-1379. Epub 2014 Mar 28. Mol Endocrinol. 2014. PMID: 24678732 Free PMC article.
-
Retinoic acid actions through mammalian nuclear receptors.Chem Rev. 2014 Jan 8;114(1):233-54. doi: 10.1021/cr400161b. Epub 2013 Dec 5. Chem Rev. 2014. PMID: 24308533 Free PMC article. Review. No abstract available.
References
-
- Britten R. J., Davidson E. H. (1969) Science 165, 349–357 - PubMed
-
- Spelsberg T. C., Steggles A. W., O'Malley B. W. (1971) J. Biol. Chem. 246, 4188–4197 - PubMed
-
- Murray M. B., Towle H. C. (1989) Mol. Endocrinol. 3, 1434–1442 - PubMed
-
- Ma J., Ptashne M. (1988) Cell 55, 443–446 - PubMed
-
- Dynlacht B. D., Hoey T., Tjian R. (1991) Cell 66, 563–576 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

