NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration
- PMID: 21067594
- PMCID: PMC2992530
- DOI: 10.1186/1750-1326-5-49
NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration
Abstract
Background: The phosphatase PTEN governs the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway which is arguably the most important pro-survival pathway in neurons. Recently, PTEN has also been implicated in multiple important CNS functions such as neuronal differentiation, plasticity, injury and drug addiction. It has been reported that loss of PTEN protein, accompanied by Akt activation, occurs under excitotoxic conditions (stroke) as well as in Alzheimer's (AD) brains. However the molecular signals and mechanism underlying PTEN loss are unknown.
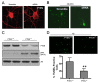
Results: In this study, we investigated redox regulation of PTEN, namely S-nitrosylation, a covalent modification of cysteine residues by nitric oxide (NO), and H2O2-mediated oxidation. We found that S-nitrosylation of PTEN was markedly elevated in brains in the early stages of AD (MCI). Surprisingly, there was no increase in the H2O2-mediated oxidation of PTEN, a modification common in cancer cell types, in the MCI/AD brains as compared to normal aged control. Using several cultured neuronal models, we further demonstrate that S-nitrosylation, in conjunction with NO-mediated enhanced ubiquitination, regulates both the lipid phosphatase activity and protein stability of PTEN. S-nitrosylation and oxidation occur on overlapping and distinct Cys residues of PTEN. The NO signal induces PTEN protein degradation via the ubiquitin-proteasome system (UPS) through NEDD4-1-mediated ubiquitination.
Conclusion: This study demonstrates for the first time that NO-mediated redox regulation is the mechanism of PTEN protein degradation, which is distinguished from the H2O2-mediated PTEN oxidation, known to only inactivate the enzyme. This novel regulatory mechanism likely accounts for the PTEN loss observed in neurodegeneration such as in AD, in which NO plays a critical pathophysiological role.
Figures



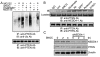
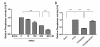

Similar articles
-
Functional interaction of phosphatase and tensin homologue (PTEN) with the E3 ligase NEDD4-1 during neuronal response to zinc.J Biol Chem. 2010 Mar 26;285(13):9847-9857. doi: 10.1074/jbc.M109.091637. Epub 2010 Jan 25. J Biol Chem. 2010. PMID: 20100827 Free PMC article.
-
Differential regulation of BACE1 expression by oxidative and nitrosative signals.Mol Neurodegener. 2011 Mar 3;6:17. doi: 10.1186/1750-1326-6-17. Mol Neurodegener. 2011. PMID: 21371311 Free PMC article.
-
iNOS Associates With Poor Survival in Melanoma: A Role for Nitric Oxide in the PI3K-AKT Pathway Stimulation and PTEN S-Nitrosylation.Front Oncol. 2021 Feb 12;11:631766. doi: 10.3389/fonc.2021.631766. eCollection 2021. Front Oncol. 2021. PMID: 33643925 Free PMC article.
-
S-Nitrosylation in neurogenesis and neuronal development.Biochim Biophys Acta. 2015 Aug;1850(8):1588-93. doi: 10.1016/j.bbagen.2014.12.013. Epub 2014 Dec 18. Biochim Biophys Acta. 2015. PMID: 25527866 Free PMC article. Review.
-
Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling.Antioxid Redox Signal. 2019 Apr 1;30(10):1331-1351. doi: 10.1089/ars.2017.7403. Epub 2018 Jan 10. Antioxid Redox Signal. 2019. PMID: 29130312 Free PMC article. Review.
Cited by
-
Modulation of AMPA Receptors by Nitric Oxide in Nerve Cells.Int J Mol Sci. 2020 Feb 1;21(3):981. doi: 10.3390/ijms21030981. Int J Mol Sci. 2020. PMID: 32024149 Free PMC article. Review.
-
Regulation by S-nitrosylation of protein post-translational modification.J Biol Chem. 2012 Feb 10;287(7):4411-8. doi: 10.1074/jbc.R111.285742. Epub 2011 Dec 6. J Biol Chem. 2012. PMID: 22147701 Free PMC article. Review.
-
Modifying PTEN recruitment promotes neuron survival, regeneration, and functional recovery after CNS injury.Cell Death Dis. 2019 Jul 29;10(8):567. doi: 10.1038/s41419-019-1802-z. Cell Death Dis. 2019. PMID: 31358730 Free PMC article.
-
PTEN S-nitrosylation by NOS1 inhibits autophagy in NPC cells.Cell Death Dis. 2019 Apr 5;10(4):306. doi: 10.1038/s41419-019-1542-0. Cell Death Dis. 2019. PMID: 30952837 Free PMC article. No abstract available.
-
PTEN degradation after ischemic stroke: a double-edged sword.Neuroscience. 2014 Aug 22;274:153-61. doi: 10.1016/j.neuroscience.2014.05.027. Epub 2014 May 27. Neuroscience. 2014. PMID: 24875179 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

