Microarray analysis and barcoded pyrosequencing provide consistent microbial profiles depending on the source of human intestinal samples
- PMID: 21257804
- PMCID: PMC3067328
- DOI: 10.1128/AEM.02477-10
Microarray analysis and barcoded pyrosequencing provide consistent microbial profiles depending on the source of human intestinal samples
Abstract
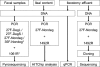
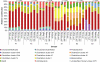
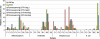
Large-scale and in-depth characterization of the intestinal microbiota necessitates application of high-throughput 16S rRNA gene-based technologies, such as barcoded pyrosequencing and phylogenetic microarray analysis. In this study, the two techniques were compared and contrasted for analysis of the bacterial composition in three fecal and three small intestinal samples from human individuals. As PCR remains a crucial step in sample preparation for both techniques, different forward primers were used for amplification to assess their impact on microbial profiling results. An average of 7,944 pyrosequences, spanning the V1 and V2 region of 16S rRNA genes, was obtained per sample. Although primer choice in barcoded pyrosequencing did not affect species richness and diversity estimates, detection of Actinobacteria strongly depended on the selected primer. Microbial profiles obtained by pyrosequencing and phylogenetic microarray analysis (HITChip) correlated strongly for fecal and ileal lumen samples but were less concordant for ileostomy effluent. Quantitative PCR was employed to investigate the deviations in profiling between pyrosequencing and HITChip analysis. Since cloning and sequencing of random 16S rRNA genes from ileostomy effluent confirmed the presence of novel intestinal phylotypes detected by pyrosequencing, especially those belonging to the Veillonella group, the divergence between pyrosequencing and the HITChip is likely due to the relatively low number of available 16S rRNA gene sequences of small intestinal origin in the DNA databases that were used for HITChip probe design. Overall, this study demonstrated that equivalent biological conclusions are obtained by high-throughput profiling of microbial communities, independent of technology or primer choice.
Figures
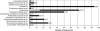
Similar articles
-
Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine.PLoS One. 2009 Aug 20;4(8):e6669. doi: 10.1371/journal.pone.0006669. PLoS One. 2009. PMID: 19693277 Free PMC article.
-
Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison.PLoS One. 2011;6(7):e22788. doi: 10.1371/journal.pone.0022788. Epub 2011 Jul 29. PLoS One. 2011. PMID: 21829515 Free PMC article.
-
Comparative analysis of Korean human gut microbiota by barcoded pyrosequencing.PLoS One. 2011;6(7):e22109. doi: 10.1371/journal.pone.0022109. Epub 2011 Jul 29. PLoS One. 2011. PMID: 21829445 Free PMC article.
-
Application of phylogenetic microarrays to interrogation of human microbiota.FEMS Microbiol Ecol. 2012 Jan;79(1):2-11. doi: 10.1111/j.1574-6941.2011.01222.x. Epub 2011 Nov 9. FEMS Microbiol Ecol. 2012. PMID: 22092522 Free PMC article. Review.
-
Experimental and analytical tools for studying the human microbiome.Nat Rev Genet. 2011 Dec 16;13(1):47-58. doi: 10.1038/nrg3129. Nat Rev Genet. 2011. PMID: 22179717 Free PMC article. Review.
Cited by
-
Draft Genome Sequence of Enterococcus sp. Strain HSIEG1, Isolated from the Human Small Intestine.Genome Announc. 2013 Dec 12;1(6):e01013-13. doi: 10.1128/genomeA.01013-13. Genome Announc. 2013. PMID: 24336366 Free PMC article.
-
Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue.Front Microbiol. 2011 Jul 13;2:149. doi: 10.3389/fmicb.2011.00149. eCollection 2011. Front Microbiol. 2011. PMID: 21808634 Free PMC article.
-
Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status.PLoS One. 2014 Apr 14;9(4):e94863. doi: 10.1371/journal.pone.0094863. eCollection 2014. PLoS One. 2014. PMID: 24733310 Free PMC article.
-
Diet, microbiota, and dysbiosis: a 'recipe' for colorectal cancer.Food Funct. 2016 Apr;7(4):1731-40. doi: 10.1039/c5fo01276g. Food Funct. 2016. PMID: 26840037 Free PMC article. Review.
-
Methods for exploring the faecal microbiome of premature infants: a review.Matern Health Neonatol Perinatol. 2021 Mar 8;7(1):11. doi: 10.1186/s40748-021-00131-9. Matern Health Neonatol Perinatol. 2021. PMID: 33685524 Free PMC article. Review.
References
-
- Baker, G. C., J. J. Smith, and D. A. Cowan. 2003. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 55:541-555. - PubMed
-
- Booijink, C. C., et al. 2010. High temporal and inter-individual variation detected in the human ileal microbiota. Environ. Microbiol. 12:3213-3227. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

