Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists
- PMID: 21264319
- PMCID: PMC3019225
- DOI: 10.1371/journal.pone.0016088
Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists
Abstract
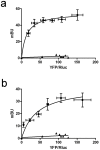
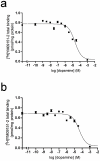
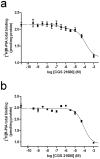
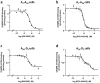
Striatal adenosine A(2A) receptors (A(2A)Rs) are highly expressed in medium spiny neurons (MSNs) of the indirect efferent pathway, where they heteromerize with dopamine D(2) receptors (D(2)Rs). A(2A)Rs are also localized presynaptically in cortico-striatal glutamatergic terminals contacting MSNs of the direct efferent pathway, where they heteromerize with adenosine A(1) receptors (A(1)Rs). It has been hypothesized that postsynaptic A(2A)R antagonists should be useful in Parkinson's disease, while presynaptic A(2A)R antagonists could be beneficial in dyskinetic disorders, such as Huntington's disease, obsessive-compulsive disorders and drug addiction. The aim or this work was to determine whether selective A(2A)R antagonists may be subdivided according to a preferential pre- versus postsynaptic mechanism of action. The potency at blocking the motor output and striatal glutamate release induced by cortical electrical stimulation and the potency at inducing locomotor activation were used as in vivo measures of pre- and postsynaptic activities, respectively. SCH-442416 and KW-6002 showed a significant preferential pre- and postsynaptic profile, respectively, while the other tested compounds (MSX-2, SCH-420814, ZM-241385 and SCH-58261) showed no clear preference. Radioligand-binding experiments were performed in cells expressing A(2A)R-D(2)R and A(1)R-A(2A)R heteromers to determine possible differences in the affinity of these compounds for different A(2A)R heteromers. Heteromerization played a key role in the presynaptic profile of SCH-442416, since it bound with much less affinity to A(2A)R when co-expressed with D(2)R than with A(1)R. KW-6002 showed the best relative affinity for A(2A)R co-expressed with D(2)R than co-expressed with A(1)R, which can at least partially explain the postsynaptic profile of this compound. Also, the in vitro pharmacological profile of MSX-2, SCH-420814, ZM-241385 and SCH-58261 was is in accordance with their mixed pre- and postsynaptic profile. On the basis of their preferential pre- versus postsynaptic actions, SCH-442416 and KW-6002 may be used as lead compounds to obtain more effective antidyskinetic and antiparkinsonian compounds, respectively.
Conflict of interest statement
Figures
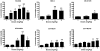
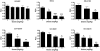

Similar articles
-
Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum.Neuropharmacology. 2011 Oct-Nov;61(5-6):967-74. doi: 10.1016/j.neuropharm.2011.06.025. Epub 2011 Jul 5. Neuropharmacology. 2011. PMID: 21752341 Free PMC article.
-
Functional changes in postsynaptic adenosine A(2A) receptors during early stages of a rat model of Huntington disease.Exp Neurol. 2011 Nov;232(1):76-80. doi: 10.1016/j.expneurol.2011.08.005. Epub 2011 Aug 16. Exp Neurol. 2011. PMID: 21867705 Free PMC article.
-
Up-regulation of striatal adenosine A(2A) receptors with iron deficiency in rats: effects on locomotion and cortico-striatal neurotransmission.Exp Neurol. 2010 Jul;224(1):292-8. doi: 10.1016/j.expneurol.2010.04.004. Epub 2010 Apr 10. Exp Neurol. 2010. PMID: 20385128 Free PMC article.
-
Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function.Neurology. 2003 Dec 9;61(11 Suppl 6):S12-8. doi: 10.1212/01.wnl.0000095205.33940.99. Neurology. 2003. PMID: 14663003 Review.
-
Adenosine-cannabinoid receptor interactions. Implications for striatal function.Br J Pharmacol. 2010 Jun;160(3):443-53. doi: 10.1111/j.1476-5381.2010.00723.x. Br J Pharmacol. 2010. PMID: 20590556 Free PMC article. Review.
Cited by
-
Connectome and molecular pharmacological differences in the dopaminergic system in restless legs syndrome (RLS): plastic changes and neuroadaptations that may contribute to augmentation.Sleep Med. 2017 Mar;31:71-77. doi: 10.1016/j.sleep.2016.06.003. Epub 2016 Jun 27. Sleep Med. 2017. PMID: 27539027 Free PMC article. Review.
-
Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum.Neuropharmacology. 2011 Oct-Nov;61(5-6):967-74. doi: 10.1016/j.neuropharm.2011.06.025. Epub 2011 Jul 5. Neuropharmacology. 2011. PMID: 21752341 Free PMC article.
-
Adenosine A(2A) Receptors and A(2A) Receptor Heteromers as Key Players in Striatal Function.Front Neuroanat. 2011 Jun 17;5:36. doi: 10.3389/fnana.2011.00036. eCollection 2011. Front Neuroanat. 2011. PMID: 21731559 Free PMC article.
-
The Role of Adenosine Tone and Adenosine Receptors in Huntington's Disease.J Caffeine Adenosine Res. 2018 Jun 1;8(2):43-58. doi: 10.1089/caff.2018.0006. J Caffeine Adenosine Res. 2018. PMID: 30023989 Free PMC article. Review.
-
Adenosine 2A Receptor Antagonists for the Treatment of Motor Symptoms in Parkinson's Disease.Mov Disord Clin Pract. 2015 Jul 25;2(4):331-340. doi: 10.1002/mdc3.12187. eCollection 2015 Dec. Mov Disord Clin Pract. 2015. PMID: 30363540 Free PMC article. Review.
References
-
- Gerfen CR. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. Amsterdam: Elsevier Academic Press; 2004. pp. 445–508.
-
- Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Arbizu J, Giménez-Amaya JM. The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci. 2002;17:51–55. - PubMed
-
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

