Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E
- PMID: 21385896
- PMCID: PMC3085722
- DOI: 10.1158/0008-5472.CAN-10-4086
Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E
Abstract
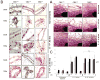
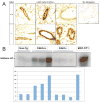
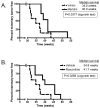
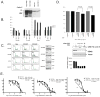
Cyclin E activates Cdk2, controls centrosome duplication, and regulates histone gene transcription. Cyclin E is deregulated in cancer and appears as low-molecular-weight (LMW) isoforms that correlate strongly with decreased survival in breast cancer patients. Transgenic mice overexpressing LMW-cyclin E have increased incidence of mammary tumors and distant metastasis when compared with mice that had full-length cyclin E. To specifically test the requirement for Cdk2 in LMW-cyclin E-mediated mammary tumorigenesis, we generated transgenic mice, which expressed LMW-cyclin E in a Cdk2-deficient background. We found that mammary gland development proceeds relatively normally in these animals, indicating that Cdk2 kinase activity is largely dispensable for this process. However, Cdk2-deficient mice were completely resistant to LMW-cyclin E-mediated mammary tumors. Cdk2 wild-type or heterozygous mice succumbed to mammary tumors with mean latencies of 16 and 19.5 months, respectively, but Cdk2 nullizygous littermates did not display tumors through 24 months. Similarly, continuous administration of two different Cdk inhibitors significantly delayed LMW-cyclin E-induced mammary tumor progression. Triple transgenic mice generated in a p53 heterozygous background also displayed no tumors. Finally, we found that Cdk2 silencing induced cell death in LMW-overexpressing breast cancer cell lines, but not in cell lines lacking LMW expression. Our findings establish a requirement for Cdk2 in LMW-cyclin E-mediated mammary tumorigenesis, arguing that human breast tumors overexpressing LMW-cyclin E are prime candidates for anti-Cdk2 therapy.
Conflict of interest statement
Conflict of interest: All authors declare no conflict of interest.
Figures

Similar articles
-
LMW-E/CDK2 deregulates acinar morphogenesis, induces tumorigenesis, and associates with the activated b-Raf-ERK1/2-mTOR pathway in breast cancer patients.PLoS Genet. 2012;8(3):e1002538. doi: 10.1371/journal.pgen.1002538. Epub 2012 Mar 29. PLoS Genet. 2012. PMID: 22479189 Free PMC article.
-
_targeting low molecular weight cyclin E (LMW-E) in breast cancer.Breast Cancer Res Treat. 2012 Apr;132(2):575-88. doi: 10.1007/s10549-011-1638-4. Epub 2011 Jun 22. Breast Cancer Res Treat. 2012. PMID: 21695458 Free PMC article.
-
Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway.Cancer Res. 2007 Aug 1;67(15):7212-22. doi: 10.1158/0008-5472.CAN-07-0599. Cancer Res. 2007. PMID: 17671189
-
Low-Molecular-Weight Cyclin E in Human Cancer: Cellular Consequences and Opportunities for _targeted Therapies.Cancer Res. 2018 Oct 1;78(19):5481-5491. doi: 10.1158/0008-5472.CAN-18-1235. Epub 2018 Sep 7. Cancer Res. 2018. PMID: 30194068 Free PMC article. Review.
-
Cyclin E and its low molecular weight forms in human cancer and as _targets for cancer therapy.Cancer Biol Ther. 2003 Jul-Aug;2(4 Suppl 1):S38-47. Cancer Biol Ther. 2003. PMID: 14508079 Review.
Cited by
-
_targeting cell-cycle machinery in cancer.Cancer Cell. 2021 Jun 14;39(6):759-778. doi: 10.1016/j.ccell.2021.03.010. Epub 2021 Apr 22. Cancer Cell. 2021. PMID: 33891890 Free PMC article. Review.
-
Hbo1 is a cyclin E/CDK2 substrate that enriches breast cancer stem-like cells.Cancer Res. 2013 Sep 1;73(17):5556-68. doi: 10.1158/0008-5472.CAN-13-0013. Epub 2013 Aug 16. Cancer Res. 2013. PMID: 23955388 Free PMC article.
-
CDK2-AP1 inhibits growth of breast cancer cells by regulating cell cycle and increasing docetaxel sensitivity in vivo and in vitro.Cancer Cell Int. 2014 Nov 30;14(1):130. doi: 10.1186/s12935-014-0130-8. eCollection 2014. Cancer Cell Int. 2014. PMID: 25550687 Free PMC article.
-
Cyclin E overexpression as a biomarker for combination treatment strategies in inflammatory breast cancer.Onco_target. 2017 Feb 28;8(9):14897-14911. doi: 10.18632/onco_target.14689. Onco_target. 2017. PMID: 28107181 Free PMC article.
-
Cytoplasmic Cyclin E Predicts Recurrence in Patients with Breast Cancer.Clin Cancer Res. 2017 Jun 15;23(12):2991-3002. doi: 10.1158/1078-0432.CCR-16-2217. Epub 2016 Nov 23. Clin Cancer Res. 2017. PMID: 27881578 Free PMC article.
References
-
- Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. - PubMed
-
- Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776–86. - PubMed
-
- Caldon CE, Sutherland RL, Musgrove EA. Cell cycle proteins in epithelial cell differentiation: Implications for breast cancer. Cell Cycle. 9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

