A new neural framework for visuospatial processing
- PMID: 21415848
- PMCID: PMC3388718
- DOI: 10.1038/nrn3008
A new neural framework for visuospatial processing
Abstract
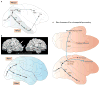
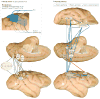
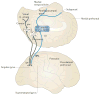
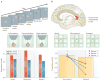
The division of cortical visual processing into distinct dorsal and ventral streams is a key framework that has guided visual neuroscience. The characterization of the ventral stream as a 'What' pathway is relatively uncontroversial, but the nature of dorsal stream processing is less clear. Originally proposed as mediating spatial perception ('Where'), more recent accounts suggest it primarily serves non-conscious visually guided action ('How'). Here, we identify three pathways emerging from the dorsal stream that consist of projections to the prefrontal and premotor cortices, and a major projection to the medial temporal lobe that courses both directly and indirectly through the posterior cingulate and retrosplenial cortices. These three pathways support both conscious and non-conscious visuospatial processing, including spatial working memory, visually guided action and navigation, respectively.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Visuomotor coordination and motor representation by human temporal lobe neurons.J Cogn Neurosci. 2012 Mar;24(3):600-10. doi: 10.1162/jocn_a_00160. Epub 2011 Nov 8. J Cogn Neurosci. 2012. PMID: 22066588
-
Neural correlates of visual form and visual spatial processing.Hum Brain Mapp. 1999;8(1):60-71. doi: 10.1002/(SICI)1097-0193(1999)8:1<60::AID-HBM5>3.0.CO;2-6. Hum Brain Mapp. 1999. PMID: 10432182 Free PMC article.
-
Distinct and overlapping fMRI activation networks for processing of novel identities and locations of objects.Eur J Neurosci. 2005 Oct;22(8):2095-105. doi: 10.1111/j.1460-9568.2005.04380.x. Eur J Neurosci. 2005. PMID: 16262647
-
'What' and 'where' in the human brain.Curr Opin Neurobiol. 1994 Apr;4(2):157-65. doi: 10.1016/0959-4388(94)90066-3. Curr Opin Neurobiol. 1994. PMID: 8038571 Review.
-
Imaging neural signatures of consciousness: 'what', 'when', 'where' and 'how' does it work?Arch Ital Biol. 2012 Jun-Sep;150(2-3):91-106. doi: 10.4449/aib.v150i2.1270. Arch Ital Biol. 2012. PMID: 23165871 Review.
Cited by
-
Prevalence of increases in functional connectivity in visual, somatosensory and language areas in congenital blindness.Front Neuroanat. 2015 Jul 1;9:86. doi: 10.3389/fnana.2015.00086. eCollection 2015. Front Neuroanat. 2015. PMID: 26190978 Free PMC article.
-
Wide-field Ca(2+) imaging reveals visually evoked activity in the retrosplenial area.Front Mol Neurosci. 2015 Jun 8;8:20. doi: 10.3389/fnmol.2015.00020. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26106292 Free PMC article.
-
Distribution of calbindin-positive neurons across areas and layers of the marmoset cerebral cortex.PLoS Comput Biol. 2024 Sep 23;20(9):e1012428. doi: 10.1371/journal.pcbi.1012428. eCollection 2024 Sep. PLoS Comput Biol. 2024. PMID: 39312590 Free PMC article.
-
Conscious experience and episodic memory: hippocampus at the crossroads.Front Psychol. 2013 May 30;4:304. doi: 10.3389/fpsyg.2013.00304. eCollection 2013. Front Psychol. 2013. PMID: 23755033 Free PMC article.
-
Functional networks and structural connectivity of visuospatial and visuoperceptual working memory.Front Hum Neurosci. 2015 Jun 11;9:340. doi: 10.3389/fnhum.2015.00340. eCollection 2015. Front Hum Neurosci. 2015. PMID: 26124716 Free PMC article.
References
-
- Ungerleider LG, Mishkin M. In: Analysis of Visual Behavior. Ingle DJ, Goodale MA, Mansfield RJW, editors. MIT Press; Cambridge, Massachusetts: 1982. pp. 549–586.
-
- Mishkin M, Ungerleider LG, Macko K. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6:414–417.
-
- Macko KA, et al. Mapping the primate visual system with [2–14C]deoxyglucose. Science. 1982;218:394–397. - PubMed
-
- Milner AD, et al. Perception and action in ‘visual form agnosia’. Brain. 1991;114:405–428. - PubMed
-
- James TW, Culham J, Humphrey GK, Milner AD, Goodale MA. Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain. 2003;126:2463–2475. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

