Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration
- PMID: 21421841
- PMCID: PMC3078381
- DOI: 10.1073/pnas.1017033108
Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration
Abstract
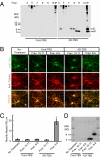
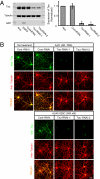
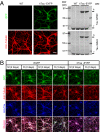
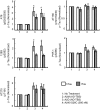
Alzheimer disease is a major cause of cognitive failure, and a pathogenically related but more subtle process accounts for many cases of mild memory symptoms in older humans. Insoluble fibrillar plaques of amyloid β-proteins (Aβ) and neurofibrillary deposits of hyperphosphorylated tau proteins are the diagnostic lesions of AD, but their temporal mechanistic relationship has long been debated. The recent recognition that small, diffusible oligomers may be the principal bioactive form of Aβ raises the key question of whether these are sufficient to initiate cytoskeletal change and neurite degeneration. A few studies have examined the effects of oligomers of synthetic Aβ peptides of one defined length at supraphysiological concentrations, but the existence of such assemblies in the AD brain is not established. Here, we isolated Aβ dimers, the most abundant form of soluble oligomer detectable in the human brain, from the cortices of typical AD subjects and found that at subnanomolar concentrations, they first induced hyperphosphorylation of tau at AD-relevant epitopes in hippocampal neurons and then disrupted the microtubule cytoskeleton and caused neuritic degeneration, all in the absence of amyloid fibrils. Application of pure, synthetic dimers confirmed the effects of the natural AD dimers, although the former were far less potent. Knocking down endogenous tau fully prevented the neuritic changes, whereas overexpressing human tau accelerated them. Coadministering Aβ N-terminal antibodies neutralized the cytoskeletal disruption. We conclude that natural dimers isolated from the AD brain are sufficient to potently induce AD-type tau phosphorylation and then neuritic dystrophy, but passive immunotherapy mitigates this.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation.J Neurosci. 2001 Feb 1;21(3):834-42. doi: 10.1523/JNEUROSCI.21-03-00834.2001. J Neurosci. 2001. PMID: 11157069 Free PMC article.
-
Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures.Neuroscience. 2002;115(1):201-11. doi: 10.1016/s0306-4522(02)00404-9. Neuroscience. 2002. PMID: 12401334
-
Fibrillar Amyloid-β Accumulation Triggers an Inflammatory Mechanism Leading to Hyperphosphorylation of the Carboxyl-Terminal End of Tau Polypeptide in the Hippocampal Formation of the 3×Tg-AD Transgenic Mouse.J Alzheimers Dis. 2016 Mar 22;52(1):243-69. doi: 10.3233/JAD-150837. J Alzheimers Dis. 2016. PMID: 27031470
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
The amyloid hypothesis of Alzheimer's disease at 25 years.EMBO Mol Med. 2016 Jun 1;8(6):595-608. doi: 10.15252/emmm.201606210. Print 2016 Jun. EMBO Mol Med. 2016. PMID: 27025652 Free PMC article. Review.
Cited by
-
Age differences in BOLD modulation to task difficulty as a function of amyloid burden.Cereb Cortex. 2024 Sep 3;34(9):bhae357. doi: 10.1093/cercor/bhae357. Cereb Cortex. 2024. PMID: 39227310 Free PMC article.
-
Neuritic Plaques - Gateways to Understanding Alzheimer's Disease.Mol Neurobiol. 2024 May;61(5):2808-2821. doi: 10.1007/s12035-023-03736-7. Epub 2023 Nov 8. Mol Neurobiol. 2024. PMID: 37940777 Free PMC article. Review.
-
Neuritic and Diffuse Plaque Associations with Memory in Non-Cognitively Impaired Elderly.J Alzheimers Dis. 2016 Jul 14;53(4):1641-52. doi: 10.3233/JAD-160365. J Alzheimers Dis. 2016. PMID: 27540968 Free PMC article.
-
The neurodegeneration in Alzheimer disease and the prion protein.Prion. 2013 Jan-Feb;7(1):60-5. doi: 10.4161/pri.23286. Epub 2013 Jan 1. Prion. 2013. PMID: 23324596 Free PMC article. Review.
-
Multi-_target Directed Donepezil-Like Ligands for Alzheimer's Disease.Front Neurosci. 2016 May 25;10:205. doi: 10.3389/fnins.2016.00205. eCollection 2016. Front Neurosci. 2016. PMID: 27252617 Free PMC article. Review.
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Dodart JC, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

