Chemically defined conditions for human iPSC derivation and culture
- PMID: 21478862
- PMCID: PMC3084903
- DOI: 10.1038/nmeth.1593
Chemically defined conditions for human iPSC derivation and culture
Abstract
We re-examine the individual components for human embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) culture and formulate a cell culture system in which all protein reagents for liquid media, attachment surfaces and splitting are chemically defined. A major improvement is the lack of a serum albumin component, as variations in either animal- or human-sourced albumin batches have previously plagued human ESC and iPSC culture with inconsistencies. Using this new medium (E8) and vitronectin-coated surfaces, we demonstrate improved derivation efficiencies of vector-free human iPSCs with an episomal approach. This simplified E8 medium should facilitate both the research use and clinical applications of human ESCs and iPSCs and their derivatives, and should be applicable to other reprogramming methods.
Figures
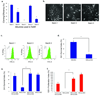
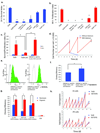
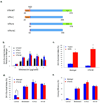
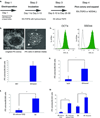
Comment in
-
Keeping things simple.Nat Methods. 2011 May;8(5):389-90. doi: 10.1038/nmeth.1598. Nat Methods. 2011. PMID: 21527929 No abstract available.
Similar articles
-
Generation of Human iPSCs by Episomal Reprogramming of Skin Fibroblasts and Peripheral Blood Mononuclear Cells.Methods Mol Biol. 2021;2239:135-151. doi: 10.1007/978-1-0716-1084-8_9. Methods Mol Biol. 2021. PMID: 33226617
-
Non-integrating episomal plasmid-based reprogramming of human amniotic fluid stem cells into induced pluripotent stem cells in chemically defined conditions.Cell Cycle. 2016;15(2):234-49. doi: 10.1080/15384101.2015.1121332. Cell Cycle. 2016. PMID: 26654216 Free PMC article.
-
Derivation, Expansion, and Motor Neuron Differentiation of Human-Induced Pluripotent Stem Cells with Non-Integrating Episomal Vectors and a Defined Xenogeneic-free Culture System.Mol Neurobiol. 2016 Apr;53(3):1589-1600. doi: 10.1007/s12035-014-9084-z. Epub 2015 Feb 10. Mol Neurobiol. 2016. PMID: 25663198
-
Evolution of induced pluripotent stem cell technology.Curr Opin Hematol. 2010 Jul;17(4):276-80. doi: 10.1097/MOH.0b013e328339f2ee. Curr Opin Hematol. 2010. PMID: 20442654 Review.
-
From skin to the treatment of diseases--the possibilities of iPS cell research in dermatology.Exp Dermatol. 2011 Jun;20(6):523-8. doi: 10.1111/j.1600-0625.2011.01282.x. Exp Dermatol. 2011. PMID: 21585557 Review.
Cited by
-
A cost-effective RNA sequencing protocol for large-scale gene expression studies.Sci Rep. 2015 Apr 1;5:9570. doi: 10.1038/srep09570. Sci Rep. 2015. PMID: 25831155 Free PMC article.
-
A facile method to establish human induced pluripotent stem cells from adult blood cells under feeder-free and xeno-free culture conditions: a clinically compliant approach.Stem Cells Transl Med. 2015 Apr;4(4):320-32. doi: 10.5966/sctm.2014-0214. Epub 2015 Mar 5. Stem Cells Transl Med. 2015. PMID: 25742692 Free PMC article.
-
Recombinant production of growth factors for application in cell culture.iScience. 2022 Sep 3;25(10):105054. doi: 10.1016/j.isci.2022.105054. eCollection 2022 Oct 21. iScience. 2022. PMID: 36157583 Free PMC article.
-
Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the _targeted locus.Cell Rep. 2015 May 12;11(6):875-883. doi: 10.1016/j.celrep.2015.04.007. Epub 2015 Apr 30. Cell Rep. 2015. PMID: 25937281 Free PMC article.
-
Feeder cells support the culture of induced pluripotent stem cells even after chemical fixation.PLoS One. 2012;7(3):e32707. doi: 10.1371/journal.pone.0032707. Epub 2012 Mar 2. PLoS One. 2012. PMID: 22396791 Free PMC article.
References
-
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. - PubMed
-
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451 141-U141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

