Anti-inflammatory activity of Chios mastic gum is associated with inhibition of TNF-alpha induced oxidative stress
- PMID: 21645369
- PMCID: PMC3127998
- DOI: 10.1186/1475-2891-10-64
Anti-inflammatory activity of Chios mastic gum is associated with inhibition of TNF-alpha induced oxidative stress
Abstract
Background: Gum of Chios mastic (Pistacia lentiscus var. chia) is a natural antimicrobial agent that has found extensive use in pharmaceutical products and as a nutritional supplement. The molecular mechanisms of its anti-inflammatory activity, however, are not clear. In this work, the potential role of antioxidant activity of Chios mastic gum has been evaluated.
Methods: Scavenging of superoxide radical was investigated by electron spin resonance and spin trapping technique using EMPO spin trap in xanthine oxidase system. Superoxide production in endothelial and smooth muscle cells stimulated with TNF-α or angiotensin II and treated with vehicle (DMSO) or mastic gum (0.1-10 μg/ml) was measured by DHE and HPLC. Cellular H2O2 was measured by Amplex Red. Inhibition of protein kinase C (PKC) with mastic gum was determined by the decrease of purified PKC activity, by inhibition of PKC activity in cellular homogenate and by attenuation of superoxide production in cells treated with PKC activator phorbol 12-myristate 13-acetate (PMA).
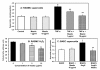
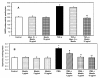
Results: Spin trapping study did not show significant scavenging of superoxide by mastic gum itself. However, mastic gum inhibited cellular production of superoxide and H2O2 in dose dependent manner in TNF-α treated rat aortic smooth muscle cells but did not affect unstimulated cells. TNF-α significantly increased the cellular superoxide production by NADPH oxidase, while mastic gum completely abolished this stimulation. Mastic gum inhibited the activity of purified PKC, decreased PKC activity in cell homogenate, and attenuated superoxide production in cells stimulated with PKC activator PMA and PKC-dependent angiotensin II in endothelial cells.
Conclusion: We suggest that mastic gum inhibits PKC which attenuates production of superoxide and H2O2 by NADPH oxidases. This antioxidant property may have direct implication to the anti-inflammatory activity of the Chios mastic gum.
Figures


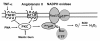
Similar articles
-
Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review.J Ethnopharmacol. 2020 May 23;254:112485. doi: 10.1016/j.jep.2019.112485. Epub 2020 Feb 22. J Ethnopharmacol. 2020. PMID: 32092498 Review.
-
Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future.Int J Mol Sci. 2023 Jul 27;24(15):12038. doi: 10.3390/ijms241512038. Int J Mol Sci. 2023. PMID: 37569412 Free PMC article. Review.
-
Current evidence on the anticancer potential of Chios mastic gum.Nutr Cancer. 2011 Nov;63(8):1174-84. doi: 10.1080/01635581.2011.607546. Epub 2011 Nov 1. Nutr Cancer. 2011. PMID: 22044444 Review.
-
Chios gum mastic: A review of its biological activities.Curr Med Chem. 2012;19(14):2292-302. doi: 10.2174/092986712800229014. Curr Med Chem. 2012. PMID: 22414110 Review.
-
Chios mastic gum extract and isolated phytosterol tirucallol exhibit anti-inflammatory activity in human aortic endothelial cells.Exp Biol Med (Maywood). 2009 May;234(5):553-61. doi: 10.3181/0811-RM-338. Epub 2009 Feb 20. Exp Biol Med (Maywood). 2009. PMID: 19234052
Cited by
-
The Use of Pistacia Lentiscus Chia Resin Versus Omeprazole in Protecting Male Rats Peptic Mucosa Against Cold Restraint Stress.J Crit Care Med (Targu Mures). 2020 May 6;6(2):100-110. doi: 10.2478/jccm-2020-0018. eCollection 2020 Apr. J Crit Care Med (Targu Mures). 2020. PMID: 32426516 Free PMC article.
-
The Short-Term Effects of Pistacia Lentiscus Oil and Sesame Oil on Liver and Kidney Pathology of Rats and Human Cancer Cell Lines.Galen Med J. 2020 Dec 29;9:e2001. doi: 10.31661/gmj.v9i0.2001. eCollection 2020. Galen Med J. 2020. PMID: 34466622 Free PMC article.
-
Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health.Nutrients. 2022 Jan 28;14(3):590. doi: 10.3390/nu14030590. Nutrients. 2022. PMID: 35276949 Free PMC article. Review.
-
Comparing the efficacy of therapeutic packages in Persian Medicine with Classical Medicine in overweight patients: a randomized clinical trial.Electron Physician. 2018 Jun 25;10(6):6892-6903. doi: 10.19082/6982. eCollection 2018 Jun. Electron Physician. 2018. PMID: 30034656 Free PMC article.
-
Direct effects of phenolic compounds on the mammary gland: In vivo and ex vivo evidence.Food Chem (Oxf). 2021 Jul 13;3:100034. doi: 10.1016/j.fochms.2021.100034. eCollection 2021 Dec 30. Food Chem (Oxf). 2021. PMID: 35415662 Free PMC article.
References
-
- Wellmann M. Pedanii Dioscuridis Anazarbei de materia medica libri quinque. Berlin: Weidmann; 1907.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

