Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II
- PMID: 21680735
- PMCID: PMC3149368
- DOI: 10.1074/jbc.M111.255331
Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II
Abstract
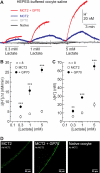
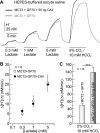
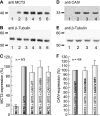
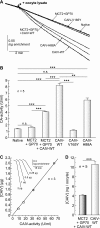
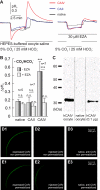
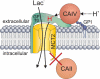
The ubiquitous enzyme carbonic anhydrase isoform II (CAII) has been shown to enhance transport activity of the proton-coupled monocarboxylate transporters MCT1 and MCT4 in a non-catalytic manner. In this study, we investigated the role of cytosolic CAII and of the extracellular, membrane-bound CA isoform IV (CAIV) on the lactate transport activity of the high-affinity monocarboxylate transporter MCT2, heterologously expressed in Xenopus oocytes. In contrast to MCT1 and MCT4, transport activity of MCT2 was not altered by CAII. However, coexpression of CAIV with MCT2 resulted in a significant increase in MCT2 transport activity when the transporter was coexpressed with its associated ancillary protein GP70 (embigin). The CAIV-mediated augmentation of MCT2 activity was independent of the catalytic activity of the enzyme, as application of the CA-inhibitor ethoxyzolamide or coexpressing the catalytically inactive mutant CAIV-V165Y did not suppress CAIV-mediated augmentation of MCT2 transport activity. Furthermore, exchange of His-88, mediating an intramolecular H(+)-shuttle in CAIV, to alanine resulted only in a slight decrease in CAIV-mediated augmentation of MCT2 activity. The data suggest that extracellular membrane-bound CAIV, but not cytosolic CAII, augments transport activity of MCT2 in a non-catalytic manner, possibly by facilitating a proton pathway other than His-88.
Figures

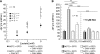

Similar articles
-
Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters.J Biol Chem. 2014 Jan 31;289(5):2765-75. doi: 10.1074/jbc.M113.537043. Epub 2013 Dec 12. J Biol Chem. 2014. PMID: 24338019 Free PMC article.
-
Membrane-anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70.J Biol Chem. 2019 Jan 11;294(2):593-607. doi: 10.1074/jbc.RA118.005536. Epub 2018 Nov 16. J Biol Chem. 2019. PMID: 30446621 Free PMC article.
-
Analysis of the binding moiety mediating the interaction between monocarboxylate transporters and carbonic anhydrase II.J Biol Chem. 2015 Feb 13;290(7):4476-86. doi: 10.1074/jbc.M114.624577. Epub 2015 Jan 5. J Biol Chem. 2015. PMID: 25561737 Free PMC article.
-
The bicarbonate transport metabolon.J Enzyme Inhib Med Chem. 2004 Jun;19(3):231-6. doi: 10.1080/14756360410001704443. J Enzyme Inhib Med Chem. 2004. PMID: 15499994 Review.
-
The role of carbonic anhydrases in renal physiology.Kidney Int. 2007 Jan;71(2):103-15. doi: 10.1038/sj.ki.5002020. Epub 2006 Dec 13. Kidney Int. 2007. PMID: 17164835 Review.
Cited by
-
pH and male fertility: making sense on pH homeodynamics throughout the male reproductive tract.Cell Mol Life Sci. 2019 Oct;76(19):3783-3800. doi: 10.1007/s00018-019-03170-w. Epub 2019 Jun 4. Cell Mol Life Sci. 2019. PMID: 31165202 Free PMC article. Review.
-
Disrupting proton dynamics and energy metabolism for cancer therapy.Nat Rev Cancer. 2013 Sep;13(9):611-23. doi: 10.1038/nrc3579. Nat Rev Cancer. 2013. PMID: 23969692 Review.
-
Functional interaction between bicarbonate transporters and carbonic anhydrase modulates lactate uptake into mouse cardiomyocytes.Pflugers Arch. 2015 Jul;467(7):1469-1480. doi: 10.1007/s00424-014-1594-z. Epub 2014 Aug 15. Pflugers Arch. 2015. PMID: 25118990
-
Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease.Pharmacol Rev. 2020 Apr;72(2):466-485. doi: 10.1124/pr.119.018762. Pharmacol Rev. 2020. PMID: 32144120 Free PMC article. Review.
-
The role of membrane acid/base transporters and carbonic anhydrases for cellular pH and metabolic processes.Front Neurosci. 2015 Jan 5;8:430. doi: 10.3389/fnins.2014.00430. eCollection 2014. Front Neurosci. 2015. PMID: 25601823 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

