And-1 is required for the stability of histone acetyltransferase Gcn5
- PMID: 21725360
- PMCID: PMC3191320
- DOI: 10.1038/onc.2011.261
And-1 is required for the stability of histone acetyltransferase Gcn5
Abstract
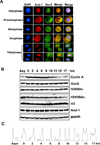
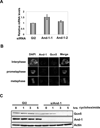
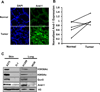
Histone acetyltransferases (HATs) have a central role in the modification of chromatin as well as in the pathogenesis of a broad set of diseases including cancers. Gcn5 is the first identified transcription-related HAT that has been implicated in the regulation of diverse cellular functions. However, how Gcn5 proteins are regulated remains largely unknown. Here we show that acidic nucleoplasmic DNA-binding protein (And-1, a high mobility group domain-containing protein) has remarkable capability to regulate the stability of Gcn5 proteins and thereby histone H3 acetylation. We find that And-1 forms a complex with both histone H3 and Gcn5. Downregulation of And-1 results in Gcn5 degradation, leading to the reduction of H3K9 and H3K56 acetylation. And-1 overexpression stabilizes Gcn5 through protein-protein interactions in vivo. Furthermore, And-1 expression is increased in cancer cells in a manner correlating with increased Gcn5 and H3K9Ac and H3K56Ac. Thus, our data reveal not only a functional link between Gcn5 and And-1 that is essential for Gcn5 protein stability and histone H3 acetylation, but also a potential role of And-1 in cancer.
Conflict of interest statement
Figures




Similar articles
-
Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation.EMBO J. 2011 Jan 19;30(2):249-62. doi: 10.1038/emboj.2010.318. Epub 2010 Dec 3. EMBO J. 2011. PMID: 21131905 Free PMC article.
-
MYC interacts with the human STAGA coactivator complex via multivalent contacts with the GCN5 and TRRAP subunits.Biochim Biophys Acta. 2014 May;1839(5):395-405. doi: 10.1016/j.bbagrm.2014.03.017. Epub 2014 Apr 3. Biochim Biophys Acta. 2014. PMID: 24705139 Free PMC article.
-
Regulation of insulin gene transcription by multiple histone acetyltransferases.DNA Cell Biol. 2012 Jan;31(1):8-14. doi: 10.1089/dna.2011.1336. Epub 2011 Jul 20. DNA Cell Biol. 2012. PMID: 21774670 Free PMC article.
-
KAT tales: Functions of Gcn5 and PCAF lysine acetyltransferases in SAGA and ATAC.J Biol Chem. 2024 Oct;300(10):107744. doi: 10.1016/j.jbc.2024.107744. Epub 2024 Sep 1. J Biol Chem. 2024. PMID: 39222683 Free PMC article. Review.
-
The Ada2/Ada3/Gcn5/Sgf29 histone acetyltransferase module.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194629. doi: 10.1016/j.bbagrm.2020.194629. Epub 2020 Sep 2. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32890768 Free PMC article. Review.
Cited by
-
Posttranslational regulation of the GCN5 and PCAF acetyltransferases.PLoS Genet. 2022 Sep 15;18(9):e1010352. doi: 10.1371/journal.pgen.1010352. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36107838 Free PMC article. Review.
-
And-1 coordinates with Claspin for efficient Chk1 activation in response to replication stress.EMBO J. 2015 Aug 4;34(15):2096-110. doi: 10.15252/embj.201488016. Epub 2015 Jun 16. EMBO J. 2015. PMID: 26082189 Free PMC article.
-
RecQL4 tethering on the pre-replicative complex induces unscheduled origin activation and replication stress in human cells.J Biol Chem. 2019 Nov 1;294(44):16255-16265. doi: 10.1074/jbc.RA119.009996. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519754 Free PMC article.
-
Histone Chaperone Nrp1 Mutation Affects the Acetylation of H3K56 in Tetrahymena thermophila.Cells. 2022 Jan 25;11(3):408. doi: 10.3390/cells11030408. Cells. 2022. PMID: 35159218 Free PMC article.
-
Regulation of histone modifying enzymes by the ubiquitin-proteasome system.Biochim Biophys Acta. 2014 Apr;1843(4):694-702. doi: 10.1016/j.bbamcr.2013.12.016. Epub 2014 Jan 3. Biochim Biophys Acta. 2014. PMID: 24389248 Free PMC article. Review.
References
-
- Armas-Pineda C, Arenas-Huertero F, Perezpenia-Diazconti M, Chico-Ponce de Leon F, Sosa-Sainz G, Lezama P, Recillas-Targa F. Journal of Experimental & Clinical Cancer Research. 2007;26:269–276. - PubMed
-
- Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Cell. 1996;84:843–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

