Changes in postnatal norepinephrine alter alpha-2 adrenergic receptor development
- PMID: 21742019
- PMCID: PMC3166411
- DOI: 10.1016/j.neuroscience.2011.06.045
Changes in postnatal norepinephrine alter alpha-2 adrenergic receptor development
Abstract
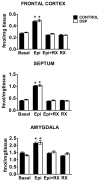
Alpha-2 adrenergic receptors (A2AR) regulate multiple brain functions and are enriched in developing brain. Studies demonstrate norepinephrine (NE) plays a role in regulating brain maturation, suggesting it is important in A2AR development. To investigate this we employed models of NE absence and excess during brain development. For decreases in NE we used N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP4), a specific noradrenergic neurotoxin. Increased noradrenergic terminal density was produced by methylazoxymethanol acetate (MAM) treatment. A2AR density was assayed with [(3)H]RX821002 autoradiography. DSP4 lesions on postnatal day (PND) 3 produce A2AR decreases in many regions by PND 5. A2AR recover to control levels by PND 15 and 25 and there is no further change in total receptor density. We also assayed A2AR in brains lesioned with DSP4 on PND 13, 23, 33 and 43 and harvested 22 days post-lesion. A2AR levels remain similar to control at each of these time points. We examined A2AR functionality and high affinity state with epinephrine-stimulated [(35)S]GTPγS and [(125)I]p-iodoclonidine autoradiography, respectively. On PND 25, control animals and animals lesioned with DSP4 on PND 3 have similar levels of [(35)S]GTPγS incorporation and no change in high affinity state. This is in contrast to increases in A2AR high affinity state produced by DSP4 lesions of mature brain. We next investigated A2AR response to increases in norepinephrine levels produced by MAM. In contrast to DSP4 lesions, increasing NE results in a large increase in A2AR. Animals treated with MAM on gestational day 14 had cortical [(3)H]RX821002 binding 100-200% greater than controls on PND 25, 35, 45, 55 and 65. These data indicate that NE regulation of A2AR differs in developing and mature brain and support the idea that NE regulates A2AR development and this has long term effects on A2AR function.
Copyright © 2011 IBRO. All rights reserved.
Figures
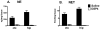
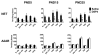
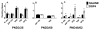
Similar articles
-
Developmental DSP4 effects on cortical Arc expression.Neurosci Lett. 2016 Apr 8;618:89-93. doi: 10.1016/j.neulet.2016.02.063. Epub 2016 Mar 3. Neurosci Lett. 2016. PMID: 26946107 Free PMC article.
-
DSP4, a selective neurotoxin for the locus coeruleus noradrenergic system. A review of its mode of action.Neurotox Res. 2015 Jan;27(1):15-30. doi: 10.1007/s12640-014-9482-z. Epub 2014 Jun 26. Neurotox Res. 2015. PMID: 24964753 Review.
-
Differential effects of neonatal norepinephrine lesions on immediate early gene expression in developing and adult rat brain.Neuroscience. 2008 Dec 10;157(4):821-32. doi: 10.1016/j.neuroscience.2008.09.036. Epub 2008 Oct 1. Neuroscience. 2008. PMID: 18938224 Free PMC article.
-
A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat.Neuroscience. 2010 Mar 10;166(1):279-91. doi: 10.1016/j.neuroscience.2009.12.027. Epub 2010 Jan 4. Neuroscience. 2010. PMID: 20045445 Free PMC article.
-
Selective Lifelong Destruction of Brain Monoaminergic Nerves Through Perinatal DSP-4 Treatment.Curr Top Behav Neurosci. 2016;29:51-71. doi: 10.1007/7854_2015_398. Curr Top Behav Neurosci. 2016. PMID: 26427851 Review.
Cited by
-
Terbutaline impairs the development of peripheral noradrenergic projections: potential implications for autism spectrum disorders and pharmacotherapy of preterm labor.Neurotoxicol Teratol. 2013 Mar-Apr;36:91-6. doi: 10.1016/j.ntt.2012.07.003. Epub 2012 Jul 16. Neurotoxicol Teratol. 2013. PMID: 22813780 Free PMC article.
-
Methodological limitations in determining astrocytic gene expression.Front Endocrinol (Lausanne). 2013 Nov 25;4:176. doi: 10.3389/fendo.2013.00176. Front Endocrinol (Lausanne). 2013. PMID: 24324456 Free PMC article. Review.
-
Developmental DSP4 effects on cortical Arc expression.Neurosci Lett. 2016 Apr 8;618:89-93. doi: 10.1016/j.neulet.2016.02.063. Epub 2016 Mar 3. Neurosci Lett. 2016. PMID: 26946107 Free PMC article.
-
DSP4, a selective neurotoxin for the locus coeruleus noradrenergic system. A review of its mode of action.Neurotox Res. 2015 Jan;27(1):15-30. doi: 10.1007/s12640-014-9482-z. Epub 2014 Jun 26. Neurotox Res. 2015. PMID: 24964753 Review.
-
The selective norepinephrine reuptake inhibitor reboxetine promotes late-stage fracture healing in mice.iScience. 2023 Aug 29;26(10):107761. doi: 10.1016/j.isci.2023.107761. eCollection 2023 Oct 20. iScience. 2023. PMID: 37720081 Free PMC article.
References
-
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. - PubMed
-
- Arnsten AFT, Steere JC, Hunt RD. The contribution of α2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry. 1996;53:448–455. - PubMed
-
- Baron BM, Seigel BW. p-[125I]Iodoclonidine, a novel radiolabeled agonist for studying central α2-adrenergic receptors. Molec Pharmacol. 1990;38:348–356. - PubMed
-
- Barturen F, Garcia-Sevilla JA. Long term treatment with desipramine increases the turnover of alpha 2-adrenoceptors in the rat brain. Mol Pharmacol. 1992;42:846–855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

