Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep
- PMID: 21784974
- PMCID: PMC3159703
- DOI: 10.4049/jimmunol.1100779
Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep
Abstract
The chorioamnionitis associated with preterm delivery is often polymicrobial with ureaplasma being the most common isolate. To evaluate interactions between the different proinflammatory mediators, we hypothesized that ureaplasma exposure would increase fetal responsiveness to LPS. Fetal sheep were given intra-amniotic (IA) injections of media (control) or Ureaplasma parvum serovar 3 either 7 or 70 d before preterm delivery. Another group received an IA injection of Escherichia coli LPS 2 d prior to delivery. To test for interactions, IA U. parvum-exposed animals were challenged with IA LPS and delivered 2 d later. All animals were delivered at 124 ± 1-d gestation (term = 150 d). Compared with the 2-d LPS exposure group, the U. parvum 70 d + LPS group had 1) decreased lung pro- and anti-inflammatory cytokine expression and 2) fewer CD3(+) T lymphocytes, CCL2(+), myeloperoxidase(+), and PU.1(+) cells in the lung. Interestingly, exposure to U. parvum for 7 d did not change responses to a subsequent IA LPS challenge, and exposure to IA U. parvum alone induced mild lung inflammation. Exposure to U. parvum increased pulmonary TGF-β1 expression but did not change mRNA expression of either the receptor TLR4 or some of the downstream mediators in the lung. Monocytes from fetal blood and lung isolated from U. parvum 70 d + LPS but not U. parvum 7 d + LPS animals had decreased in vitro responsiveness to LPS. These results are consistent with the novel finding of downregulation of LPS responses by chronic but not acute fetal exposures to U. parvum. The findings increase our understanding of how chorioamnionitis-exposed preterm infants may respond to lung injury and postnatal nosocomial infections.
Conflict of interest statement
None of the authors have a commercial interest in any entity related to subject of the manuscript or have a conflict of interest relative to the manuscript.
Figures

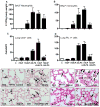
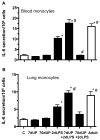
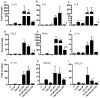
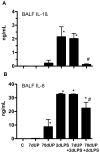
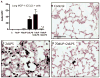
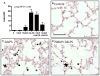
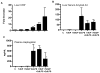
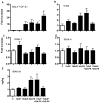
Similar articles
-
Pulmonary Consequences of Prenatal Inflammatory Exposures: Clinical Perspective and Review of Basic Immunological Mechanisms.Front Immunol. 2020 Jun 19;11:1285. doi: 10.3389/fimmu.2020.01285. eCollection 2020. Front Immunol. 2020. PMID: 32636848 Free PMC article. Review.
-
Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep.Am J Obstet Gynecol. 2013 May;208(5):399.e1-8. doi: 10.1016/j.ajog.2013.02.018. Epub 2013 Feb 11. Am J Obstet Gynecol. 2013. PMID: 23410690 Free PMC article.
-
Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum.Am J Physiol Lung Cell Mol Physiol. 2010 Dec;299(6):L852-60. doi: 10.1152/ajplung.00183.2010. Epub 2010 Oct 8. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20935228 Free PMC article.
-
Pulmonary vascular and alveolar development in preterm lambs chronically colonized with Ureaplasma parvum.Am J Physiol Lung Cell Mol Physiol. 2010 Aug;299(2):L232-41. doi: 10.1152/ajplung.00369.2009. Epub 2010 May 21. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20495079 Free PMC article.
-
Ureaplasma parvum meningitis following atypical choroid plexus papilloma resection in an adult patient: a case report and literature review.BMC Infect Dis. 2021 Dec 20;21(1):1276. doi: 10.1186/s12879-021-06975-y. BMC Infect Dis. 2021. PMID: 34930148 Free PMC article. Review.
Cited by
-
Pulmonary Consequences of Prenatal Inflammatory Exposures: Clinical Perspective and Review of Basic Immunological Mechanisms.Front Immunol. 2020 Jun 19;11:1285. doi: 10.3389/fimmu.2020.01285. eCollection 2020. Front Immunol. 2020. PMID: 32636848 Free PMC article. Review.
-
Gut and Lung Microbiota in Preterm Infants: Immunological Modulation and Implication in Neonatal Outcomes.Front Immunol. 2019 Dec 12;10:2910. doi: 10.3389/fimmu.2019.02910. eCollection 2019. Front Immunol. 2019. PMID: 31921169 Free PMC article. Review.
-
Microbial signatures in amniotic fluid at preterm birth and association with bronchopulmonary dysplasia.Respir Res. 2023 Oct 16;24(1):248. doi: 10.1186/s12931-023-02560-w. Respir Res. 2023. PMID: 37845700 Free PMC article.
-
Perinatal Ureaplasma Exposure Is Associated With Increased Risk of Late Onset Sepsis and Imbalanced Inflammation in Preterm Infants and May Add to Lung Injury.Front Cell Infect Microbiol. 2019 Apr 2;9:68. doi: 10.3389/fcimb.2019.00068. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31001484 Free PMC article.
-
Effects of multiple pro-inflammatory stimuli in utero on the ileum of extremely premature ovine fetuses.Front Immunol. 2023 May 19;14:1150208. doi: 10.3389/fimmu.2023.1150208. eCollection 2023. Front Immunol. 2023. PMID: 37275869 Free PMC article.
References
-
- Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127:146–157. - PubMed
-
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. - PubMed
-
- Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110, e111–117. - PubMed
-
- Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. - PubMed
-
- Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

