Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease
- PMID: 21878620
- PMCID: PMC3199456
- DOI: 10.1074/jbc.M111.290114
Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease
Abstract
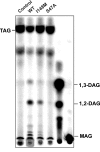
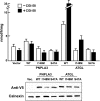
A genetic variant of PNPLA3 (patatin-like phospholipase domain-containing 3; PNPLA3-I148M), a serine protease of unknown function, is associated with accumulation of triacylglycerol (TAG) in the liver. To determine the biological substrates of PNPLA3 and the effect of the I148M substitution on enzymatic activity and substrate specificity, we purified and characterized recombinant human PNPLA3 and PNPLA3-I148M. Maximal hydrolytic activity of PNPLA3 was observed against the three major glycerolipids, TAG, diacylglycerol, and monoacylglycerol, with a strong preference for oleic acid as the acyl moiety. Substitution of methionine for isoleucine at position 148 markedly decreased the V(max) of the enzyme for glycerolipids but had only a modest effect on the K(m). Purified PNPLA3 also catalyzed the hydrolysis of oleoyl-CoA, but the V(max) was 100-fold lower for oleoyl-CoA than for triolein. The thioesterase activity required the catalytic serine but was only modestly decreased by the I148M substitution. The enzyme had little or no hydrolytic activity against the other lipid substrates tested, including phospholipids, cholesteryl ester, and retinyl esters. Neither the wild-type nor mutant enzyme catalyzed transfer of oleic acid from oleoyl-CoA to glycerophosphate, lysophosphatidic acid, or diacylglycerol, suggesting that the enzyme does not promote de novo TAG synthesis. Taken together, our results are consistent with the notion that PNPLA3 plays a role in the hydrolysis of glycerolipids and that the I148M substitution causes a loss of function, although we cannot exclude the possibility that the enzyme has additional substrates or activities.
Figures
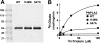
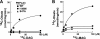


Similar articles
-
A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis.J Biol Chem. 2010 Feb 26;285(9):6706-15. doi: 10.1074/jbc.M109.064501. Epub 2009 Dec 23. J Biol Chem. 2010. PMID: 20034933 Free PMC article.
-
PNPLA3, the triacylglycerol synthesis/hydrolysis/storage dilemma, and nonalcoholic fatty liver disease.World J Gastroenterol. 2012 Nov 14;18(42):6018-26. doi: 10.3748/wjg.v18.i42.6018. World J Gastroenterol. 2012. PMID: 23155331 Free PMC article.
-
Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis.J Clin Invest. 2012 Nov;122(11):4130-44. doi: 10.1172/JCI65179. J Clin Invest. 2012. PMID: 23023705 Free PMC article.
-
PNPLA3 I148M variant in nonalcoholic fatty liver disease: demographic and ethnic characteristics and the role of the variant in nonalcoholic fatty liver fibrosis.World J Gastroenterol. 2015 Jan 21;21(3):794-802. doi: 10.3748/wjg.v21.i3.794. World J Gastroenterol. 2015. PMID: 25624712 Free PMC article. Review.
-
The role of PNPLA3 in health and disease.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):900-906. doi: 10.1016/j.bbalip.2018.06.018. Epub 2018 Jun 20. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 29935383 Review.
Cited by
-
FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling.Cell Metab. 2012 Mar 7;15(3):279-91. doi: 10.1016/j.cmet.2011.12.018. Cell Metab. 2012. PMID: 22405066 Free PMC article. Review.
-
Characterizing the mechanism behind the progression of NAFLD to hepatocellular carcinoma.Hepat Oncol. 2020 Dec 29;7(4):HEP36. doi: 10.2217/hep-2020-0017. Hepat Oncol. 2020. PMID: 33680428 Free PMC article. Review.
-
Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats.Hepatology. 2013 May;57(5):1763-72. doi: 10.1002/hep.26170. Epub 2013 Jan 25. Hepatology. 2013. PMID: 23175050 Free PMC article.
-
Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes.Nat Metab. 2019 May;1(5):560-569. doi: 10.1038/s42255-019-0066-3. Epub 2019 May 6. Nat Metab. 2019. PMID: 31497752 Free PMC article.
-
Dissociation of Fatty Liver and Insulin Resistance in I148M PNPLA3 Carriers: Differences in Diacylglycerol (DAG) FA18:1 Lipid Species as a Possible Explanation.Nutrients. 2018 Sep 17;10(9):1314. doi: 10.3390/nu10091314. Nutrients. 2018. PMID: 30227635 Free PMC article.
References
-
- Browning J. D., Szczepaniak L. S., Dobbins R., Nuremberg P., Horton J. D., Cohen J. C., Grundy S. M., Hobbs H. H. (2004) Hepatology 40, 1387–1395 - PubMed
-
- Neuschwander-Tetri B. A., Caldwell S. H. (2003) Hepatology 37, 1202–1219 - PubMed
-
- Sookoian S., Pirola C. J. (2011) Hepatology 53, 1883–1894 - PubMed
-
- Tian C., Stokowski R. P., Kershenobich D., Ballinger D. G., Hinds D. A. (2010) Nat. Genet. 42, 21–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

