Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA
- PMID: 21890902
- PMCID: PMC3241667
- DOI: 10.1093/nar/gkr695
Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA
Abstract
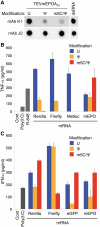
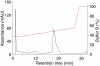
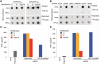
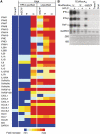
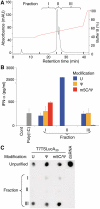
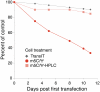
In vitro-transcribed mRNA has great therapeutic potential to transiently express the encoded protein without the adverse effects of viral and DNA-based constructs. Mammalian cells, however, contain RNA sensors of the innate immune system that must be considered in the generation of therapeutic RNA. Incorporation of modified nucleosides both reduces innate immune activation and increases translation of mRNA, but residual induction of type I interferons (IFNs) and proinflammatory cytokines remains. We identify that contaminants, including double-stranded RNA, in nucleoside-modified in vitro-transcribed RNA are responsible for innate immune activation and their removal by high performance liquid chromatography (HPLC) results in mRNA that does not induce IFNs and inflammatory cytokines and is translated at 10- to 1000-fold greater levels in primary cells. Although unmodified mRNAs were translated significantly better following purification, they still induced high levels of cytokine secretion. HPLC purified nucleoside-modified mRNA is a powerful vector for applications ranging from ex vivo stem cell generation to in vivo gene therapy.
Figures
Similar articles
-
Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability.Mol Ther. 2008 Nov;16(11):1833-40. doi: 10.1038/mt.2008.200. Epub 2008 Sep 16. Mol Ther. 2008. PMID: 18797453 Free PMC article.
-
N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice.J Control Release. 2015 Nov 10;217:337-44. doi: 10.1016/j.jconrel.2015.08.051. Epub 2015 Sep 3. J Control Release. 2015. PMID: 26342664
-
Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin.Mol Ther. 2012 May;20(5):948-53. doi: 10.1038/mt.2012.7. Epub 2012 Feb 14. Mol Ther. 2012. PMID: 22334017 Free PMC article.
-
The Epitranscriptome in Translation Regulation.Cold Spring Harb Perspect Biol. 2019 Aug 1;11(8):a032623. doi: 10.1101/cshperspect.a032623. Cold Spring Harb Perspect Biol. 2019. PMID: 30037968 Free PMC article. Review.
-
In vitro production of synthetic viral RNAs and their delivery into mammalian cells and the application of viral RNAs in the study of innate interferon responses.Methods. 2020 Nov 1;183:21-29. doi: 10.1016/j.ymeth.2019.10.013. Epub 2019 Nov 1. Methods. 2020. PMID: 31682923 Review.
Cited by
-
Applications of microfluidics in mRNA vaccine development: A review.Biomicrofluidics. 2024 Nov 14;18(6):061502. doi: 10.1063/5.0228447. eCollection 2024 Dec. Biomicrofluidics. 2024. PMID: 39553921 Review.
-
Bioinformatics analysis to design a multi-epitope mRNA vaccine against S. agalactiae exploiting pathogenic proteins.Sci Rep. 2024 Nov 16;14(1):28294. doi: 10.1038/s41598-024-79503-y. Sci Rep. 2024. PMID: 39550419 Free PMC article.
-
Navigating the intricate in-vivo journey of lipid nanoparticles tailored for the _targeted delivery of RNA therapeutics: a quality-by-design approach.J Nanobiotechnology. 2024 Nov 14;22(1):710. doi: 10.1186/s12951-024-02972-w. J Nanobiotechnology. 2024. PMID: 39543630 Free PMC article. Review.
-
Synthetic circRNA therapeutics: innovations, strategies, and future horizons.MedComm (2020). 2024 Nov 9;5(11):e720. doi: 10.1002/mco2.720. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39525953 Free PMC article. Review.
-
Exploring the Impact of In Vitro-Transcribed mRNA Impurities on Cellular Responses.Anal Chem. 2024 Nov 5;96(44):17789-17799. doi: 10.1021/acs.analchem.4c04162. Epub 2024 Oct 24. Anal Chem. 2024. PMID: 39445393
References
-
- Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem. Biophys. Res. Commun. 2010;394:189–193. - PubMed
-
- Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

