The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration
- PMID: 21911253
- PMCID: PMC3210474
- DOI: 10.1016/j.biomaterials.2011.08.019
The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration
Abstract
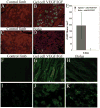
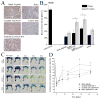
Many cell types of therapeutic interest, including myoblasts, exhibit reduced engraftment if cultured prior to transplantation. This study investigated whether polymeric scaffolds that direct cultured myoblasts to migrate outwards and repopulate the host damaged tissue, in concert with release of angiogenic factors designed to enhance revascularizaton of the regenerating tissue, would enhance the efficacy of this cell therapy and lead to functional muscle regeneration. This was investigated in the context of a severe injury to skeletal muscle tissue involving both myotoxin-mediated direct damage and induction of regional ischemia. Local and sustained release of VEGF and IGF-1 from macroporous scaffolds used to transplant and disperse cultured myogenic cells significantly enhanced their engraftment, limited fibrosis, and accelerated the regenerative process. This resulted in increased muscle mass and, improved contractile function. These results demonstrate the importance of finely controlling the microenvironment of transplanted cells in the treatment of severe muscle damage.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


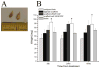
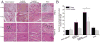
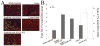
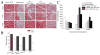
Similar articles
-
Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold.Mol Ther. 2014 Aug;22(8):1441-1449. doi: 10.1038/mt.2014.78. Epub 2014 Apr 28. Mol Ther. 2014. PMID: 24769909 Free PMC article.
-
VEGF and IGF Delivered from Alginate Hydrogels Promote Stable Perfusion Recovery in Ischemic Hind Limbs of Aged Mice and Young Rabbits.J Vasc Res. 2017;54(5):288-298. doi: 10.1159/000479869. Epub 2017 Sep 21. J Vasc Res. 2017. PMID: 28930755 Free PMC article.
-
Combining a micro/nano-hierarchical scaffold with cell-printing of myoblasts induces cell alignment and differentiation favorable to skeletal muscle tissue regeneration.Biofabrication. 2016 Sep 16;8(3):035021. doi: 10.1088/1758-5090/8/3/035021. Biofabrication. 2016. PMID: 27634918
-
Engineered matrices for skeletal muscle satellite cell engraftment and function.Matrix Biol. 2017 Jul;60-61:96-109. doi: 10.1016/j.matbio.2016.06.001. Epub 2016 Jun 4. Matrix Biol. 2017. PMID: 27269735 Free PMC article. Review.
-
Biomaterial-based delivery for skeletal muscle repair.Adv Drug Deliv Rev. 2015 Apr;84:188-97. doi: 10.1016/j.addr.2014.09.008. Epub 2014 Sep 28. Adv Drug Deliv Rev. 2015. PMID: 25271446 Free PMC article. Review.
Cited by
-
Poly(2-oxazoline) Matrices with Temperature-Dependent Solubility-Interactions with Water and Use for Cell Culture.Materials (Basel). 2020 Jun 13;13(12):2702. doi: 10.3390/ma13122702. Materials (Basel). 2020. PMID: 32545841 Free PMC article.
-
Biomaterial delivery of morphogens to mimic the natural healing cascade in bone.Adv Drug Deliv Rev. 2012 Sep;64(12):1257-76. doi: 10.1016/j.addr.2012.05.006. Epub 2012 May 22. Adv Drug Deliv Rev. 2012. PMID: 22626978 Free PMC article. Review.
-
Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo.Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5508-13. doi: 10.1073/pnas.1402723111. Epub 2014 Mar 31. Proc Natl Acad Sci U S A. 2014. PMID: 24706792 Free PMC article.
-
Regenerative medicine for skeletal muscle loss: a review of current tissue engineering approaches.J Mater Sci Mater Med. 2021 Jan 21;32(1):15. doi: 10.1007/s10856-020-06476-5. J Mater Sci Mater Med. 2021. PMID: 33475855 Free PMC article. Review.
-
Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors.Ann Biomed Eng. 2016 Jun;44(6):1908-20. doi: 10.1007/s10439-016-1594-6. Epub 2016 Mar 23. Ann Biomed Eng. 2016. PMID: 27009085 Free PMC article.
References
-
- Lubeck DP. The costs of musculoskeletal disease: health needs assessment and health economics. Best Pract Res Clin Rheumatol. 2003;17:529–39. - PubMed
-
- McClatchey KD. Musculoskeletal conditions affect millions. Arch Pathol Lab Med. 2004;128:480. - PubMed
-
- Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–6. - PubMed
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

