Inhibition of Notch signaling affects hepatic oval cell response in rat model of 2AAF-PH
- PMID: 21927552
- PMCID: PMC3172811
- DOI: 10.2147/HMER.S12368
Inhibition of Notch signaling affects hepatic oval cell response in rat model of 2AAF-PH
Abstract
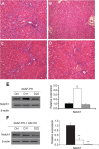
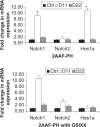
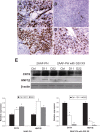
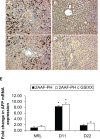
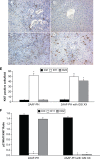
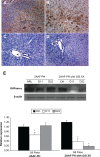
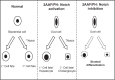
BACKGROUND AND AIMS: Activation of the oval cell compartment occurs in the liver when hepatocytes are functionally compromised and/or unable to divide. Our goal was to investigate the systemic signals responsible for determining the efficiency of oval cell-mediated liver regeneration, focusing on the Notch signaling cascade. METHODS: The established oval cell induction protocol of 2-acetylaminofluorine (2-AAF) implantation followed by 70% surgical resection of the liver (partial hepatectomy, PH) was employed in a rat model. This oval cell induction model was further combined with injections of a γ-secretase inhibitor (GSI XX) to examine the effects of Notch inhibition on oval cell-aided regeneration of the liver. RESULTS: Notch signaling was found to be upregulated at the peak of oval cell induction during 2AAF-PH alone. Treatment with GSI XX led to interruption of the Notch signal, as shown by a decrease in expression of Hes1. While there was a robust oval cell response seen at day 11 post-PH, there was a measurable delay in differentiation when Notch was inhibited. This was confirmed morphologically as well as by immunohistochemistry for the oval cell markers, α-fetoprotein, OV-6, and CK19. The hepatocytes seen at day 22 demonstrated an enhanced hepatocellular mitoinhibition index (p21(Waf1)/Ki67), suggestive of dysregulated proliferation and cell cycle progression. Moreover, these hepatocytes exhibited decreased expression of hepatocyte functional markers, such as cytochrome P450 and glucose-6-phosphatase-α. CONCLUSIONS: Taken together, these results identify the Notch signaling pathway as a potent regulator of differentiation and proliferation in oval cells, which is necessary for functional for repair of the liver by oval cells.
Figures
Similar articles
-
Cells responsible for liver mass regeneration in rats with 2-acetylaminofluorene/partial hepatectomy injury.J Biomed Sci. 2018 Apr 25;25(1):39. doi: 10.1186/s12929-018-0441-5. J Biomed Sci. 2018. PMID: 29695258 Free PMC article.
-
Characterization and enrichment of hepatic progenitor cells in adult rat liver.World J Gastroenterol. 2004 May 15;10(10):1480-6. doi: 10.3748/wjg.v10.i10.1480. World J Gastroenterol. 2004. PMID: 15133858 Free PMC article.
-
The role of the Wnt family of secreted proteins in rat oval "stem" cell-based liver regeneration: Wnt1 drives differentiation.Am J Pathol. 2010 Jun;176(6):2732-42. doi: 10.2353/ajpath.2010.080486. Epub 2010 Apr 22. Am J Pathol. 2010. PMID: 20413689 Free PMC article.
-
Hepatic oval 'stem' cell in liver regeneration.Semin Cell Dev Biol. 2002 Dec;13(6):405-9. doi: 10.1016/s1084952102001271. Semin Cell Dev Biol. 2002. PMID: 12468240 Review.
-
The role of hepatocytes and oval cells in liver regeneration and repopulation.Mech Dev. 2003 Jan;120(1):117-30. doi: 10.1016/s0925-4773(02)00338-6. Mech Dev. 2003. PMID: 12490302 Review.
Cited by
-
Signals and cells involved in regulating liver regeneration.Cells. 2012 Dec 13;1(4):1261-92. doi: 10.3390/cells1041261. Cells. 2012. PMID: 24710554 Free PMC article.
-
Hepatic progenitor cell activation in liver repair.Liver Res. 2017 Sep;1(2):81-87. doi: 10.1016/j.livres.2017.08.002. Epub 2017 Aug 9. Liver Res. 2017. PMID: 29276644 Free PMC article.
-
The diversity and plasticity of adult hepatic progenitor cells and their niche.Liver Int. 2017 Sep;37(9):1260-1271. doi: 10.1111/liv.13377. Epub 2017 Feb 23. Liver Int. 2017. PMID: 28135758 Free PMC article. Review.
-
Vascular patterning sets the stage for macro and micro hepatic architecture.Dev Dyn. 2015 Mar;244(3):497-506. doi: 10.1002/dvdy.24222. Epub 2014 Nov 18. Dev Dyn. 2015. PMID: 25370311 Free PMC article. Review.
-
Notch Inhibition Promotes Differentiation of Liver Progenitor Cells into Hepatocytes via sox9b Repression in Zebrafish.Stem Cells Int. 2019 Mar 12;2019:8451282. doi: 10.1155/2019/8451282. eCollection 2019. Stem Cells Int. 2019. PMID: 30992706 Free PMC article.
References
-
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Meile L, Osborne BA. Arbiter of differentiation: Notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. - PubMed
-
- Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: Evidence and speculation. Blood. 1999;93:2431–2448. - PubMed
-
- Weinmaster G. Notch signal transduction: A real rip and more. Curr Opin Genet Dev. 2000;10:363–369. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

