Alternative splicing produces Nanog protein variants with different capacities for self-renewal and pluripotency in embryonic stem cells
- PMID: 21969378
- PMCID: PMC3234911
- DOI: 10.1074/jbc.M111.290189
Alternative splicing produces Nanog protein variants with different capacities for self-renewal and pluripotency in embryonic stem cells
Abstract
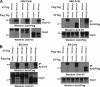
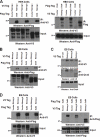
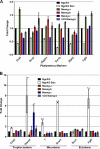
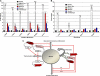
Embryonic stem (ES) cells are distinguished by their ability to undergo unlimited self-renewal although retaining pluripotency, the capacity to specify cells of all germ layers. Alternative splicing contributes to these biological processes by vastly increasing the protein coding repertoire, enabling genes to code for novel variants that may confer different biological functions. The homeodomain transcription factor Nanog acts collaboratively with core factors Oct4 and Sox2 to govern the maintenance of pluripotency. We have discovered that Nanog is regulated by alternative splicing. Two novel exons and six subexons have been identified that extend the known Nanog gene structure and protein coding capacity. Alternative splicing results in two novel Nanog protein variants with attenuated capacities for self-renewal and pluripotency in ES cells. Our previous results have implicated the C-terminal domain, including the tryptophan-rich (WR) domain of Nanog, to be important for the function of Nanog (Wang, J., Levasseur, D. N., and Orkin, S. H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6326-6331). Using point mutation analyses, serine 2 (Ser-2) of Nanog has been identified as critical for ES cell self-renewal and for stabilizing a pluripotent gene signature. An inducible conditional knock-out was created to test the ability of new Nanog variants to genetically complement Nanog null ES cells. These results reveal for the first time an expanded Nanog protein coding capacity. We further reveal that a short region of the N-terminal domain and a single phosphorylatable Ser-2 is essential for the maintenance of self-renewal and pluripotency, demonstrating that this region of the protein is highly regulated.
Figures




Similar articles
-
The C-terminal pentapeptide of Nanog tryptophan repeat domain interacts with Nac1 and regulates stem cell proliferation but not pluripotency.J Biol Chem. 2009 Jun 12;284(24):16071-16081. doi: 10.1074/jbc.M109.005041. Epub 2009 Apr 14. J Biol Chem. 2009. PMID: 19366700 Free PMC article.
-
Aromatic residues in the C-terminal domain 2 are required for Nanog to mediate LIF-independent self-renewal of mouse embryonic stem cells.J Biol Chem. 2008 Feb 22;283(8):4480-9. doi: 10.1074/jbc.M706009200. Epub 2007 Dec 17. J Biol Chem. 2008. PMID: 18086680
-
Structure-based discovery of NANOG variant with enhanced properties to promote self-renewal and reprogramming of pluripotent stem cells.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4666-71. doi: 10.1073/pnas.1502855112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825768 Free PMC article.
-
Concise review: pursuing self-renewal and pluripotency with the stem cell factor Nanog.Stem Cells. 2013 Jul;31(7):1227-36. doi: 10.1002/stem.1384. Stem Cells. 2013. PMID: 23653415 Free PMC article. Review.
-
Nanog and transcriptional networks in embryonic stem cell pluripotency.Cell Res. 2007 Jan;17(1):42-9. doi: 10.1038/sj.cr.7310125. Cell Res. 2007. PMID: 17211451 Review.
Cited by
-
Alternative splicing switching in stem cell lineages.Front Biol (Beijing). 2013 Feb 1;8(1):50-59. doi: 10.1007/s11515-012-1198-y. Front Biol (Beijing). 2013. PMID: 23399987 Free PMC article.
-
How microRNAs facilitate reprogramming to pluripotency.J Cell Sci. 2012 Sep 15;125(Pt 18):4179-87. doi: 10.1242/jcs.095968. Epub 2012 Oct 17. J Cell Sci. 2012. PMID: 23077173 Free PMC article. Review.
-
Pou5f1 and Nanog Are Reliable Germ Cell-Specific Genes in Gonad of a Protogynous Hermaphroditic Fish, Orange-Spotted Grouper (Epinephelus coioides).Genes (Basel). 2021 Dec 29;13(1):79. doi: 10.3390/genes13010079. Genes (Basel). 2021. PMID: 35052423 Free PMC article.
-
Genome editing demonstrates that the -5 kb Nanog enhancer regulates Nanog expression by modulating RNAPII initiation and/or recruitment.J Biol Chem. 2021 Jan-Jun;296:100189. doi: 10.1074/jbc.RA120.015152. Epub 2020 Dec 20. J Biol Chem. 2021. PMID: 33334884 Free PMC article.
-
RNA-binding proteins in pluripotency, differentiation, and reprogramming.Front Biol (Beijing). 2014 Oct;9(5):389-409. doi: 10.1007/s11515-014-1326-y. Front Biol (Beijing). 2014. PMID: 25554730 Free PMC article.
References
-
- Evans M. J., Kaufman M. H. (1981) Nature 292, 154–156 - PubMed
-
- Orkin S. H., Wang J., Kim J., Chu J., Rao S., Theunissen T. W., Shen X., Levasseur D. N. (2008) Cold Spring Harbor Symp. Quant. Biol. 73, 195–202 - PubMed
-
- Wang J., Rao S., Chu J., Shen X., Levasseur D. N., Theunissen T. W., Orkin S. H. (2006) Nature 444, 364–368 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

