AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian _target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways
- PMID: 21993994
- PMCID: PMC3269553
- DOI: 10.1002/hep.24736
AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian _target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways
Abstract
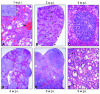
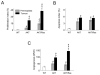
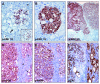
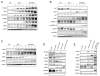
Activation of v-akt murine thymoma viral oncogene homolog (AKT) and Ras pathways is often implicated in carcinogenesis. However, the oncogenic cooperation between these two cascades in relationship to hepatocellular carcinoma (HCC) development remains undetermined. To investigate this issue, we generated a mouse model characterized by combined overexpression of activated forms of AKT and neuroblastoma Ras viral oncogene homolog (N-Ras) protooncogenes in the liver by way of hydrodynamic gene transfer. The molecular mechanisms underlying crosstalk between AKT and N-Ras were assessed in the mouse model and further evaluated in human and murine HCC cell lines. We found that coexpression of AKT and N-Ras resulted in a dramatic acceleration of liver tumor development when compared with mice overexpressing AKT alone, whereas N-Ras alone did not lead to tumor formation. At the cellular level, concomitant up-regulation of AKT and N-Ras resulted in increased proliferation and microvascularization when compared with AKT-injected mice. Mechanistic studies suggested that accelerated hepatocarcinogenesis driven by AKT and N-Ras resulted from a strong activation of mammalian _target of rapamycin complex 1 (mTORC1). Furthermore, elevated expression of FOXM1/SKP2 and c-Myc also contributed to rapid tumor growth in AKT/Ras mice, yet by way of mTORC1-independent mechanisms. The biological effects of coactivation of AKT and N-Ras were then recapitulated in vitro using HCC cell lines, which supports the functional significance of mTORC1, FOXM1/SKP2, and c-Myc signaling cascades in mediating AKT and N-Ras-induced liver tumor development.
Conclusion: Our data demonstrate the in vivo crosstalk between the AKT and Ras pathways in promoting liver tumor development, and the pivotal role of mTORC1-dependent and independent pathways in mediating AKT and Ras induced hepatocarcinogenesis.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures



Similar articles
-
4EBP1/eIF4E and p70S6K/RPS6 axes play critical and distinct roles in hepatocarcinogenesis driven by AKT and N-Ras proto-oncogenes in mice.Hepatology. 2015 Jan;61(1):200-13. doi: 10.1002/hep.27396. Epub 2014 Nov 25. Hepatology. 2015. PMID: 25145583 Free PMC article.
-
E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer.Gastroenterology. 2008 Oct;135(4):1322-32. doi: 10.1053/j.gastro.2008.07.012. Epub 2008 Jul 17. Gastroenterology. 2008. PMID: 18722373 Free PMC article.
-
V-AKT murine thymoma viral oncogene homolog/mammalian _target of rapamycin activation induces a module of metabolic changes contributing to growth in insulin-induced hepatocarcinogenesis.Hepatology. 2012 May;55(5):1473-84. doi: 10.1002/hep.25600. Epub 2012 Apr 4. Hepatology. 2012. PMID: 22271091
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
[EEF1A2 inhibits the p53 function in hepatocellular carcinoma via PI3K/AKT/mTOR-dependent stabilization of MDM4].Pathologe. 2014 Nov;35 Suppl 2:177-84. doi: 10.1007/s00292-014-2007-y. Pathologe. 2014. PMID: 25394965 Review. German.
Cited by
-
ACSL6-activated IL-18R1-NF-κB promotes IL-18-mediated tumor immune evasion and tumor progression.Sci Adv. 2024 Sep 20;10(38):eadp0719. doi: 10.1126/sciadv.adp0719. Epub 2024 Sep 18. Sci Adv. 2024. PMID: 39292786 Free PMC article.
-
EEF1A2 inactivates p53 by way of PI3K/AKT/mTOR-dependent stabilization of MDM4 in hepatocellular carcinoma.Hepatology. 2014 May;59(5):1886-99. doi: 10.1002/hep.26954. Epub 2014 Mar 27. Hepatology. 2014. PMID: 24285179 Free PMC article.
-
CD300ld on neutrophils is required for tumour-driven immune suppression.Nature. 2023 Sep;621(7980):830-839. doi: 10.1038/s41586-023-06511-9. Epub 2023 Sep 6. Nature. 2023. PMID: 37674079
-
mTORC1 Promotes ARID1A Degradation and Oncogenic Chromatin Remodeling in Hepatocellular Carcinoma.Cancer Res. 2021 Nov 15;81(22):5652-5665. doi: 10.1158/0008-5472.CAN-21-0206. Epub 2021 Aug 24. Cancer Res. 2021. PMID: 34429326 Free PMC article.
-
Anti-tumor Efficiency of Lipid-coated Cisplatin Nanoparticles Co-loaded with MicroRNA-375.Theranostics. 2016 Jan 1;6(1):142-54. doi: 10.7150/thno.13130. eCollection 2016. Theranostics. 2016. PMID: 26722380 Free PMC article.
References
-
- Bruix J, Boix L, Sala M, et al. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. - PubMed
-
- Spangenberg HC, Thimme R, Blum HE. _targeted therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2009;6:423–432. - PubMed
-
- Calvisi DF, Ladu S, Gorden A, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
