Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years
- PMID: 22048172
- PMCID: PMC3338933
- DOI: 10.4161/hv.7.12.18282
Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years
Abstract
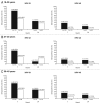
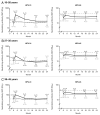
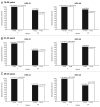
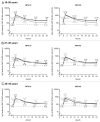
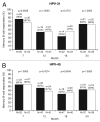
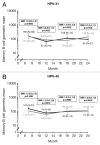
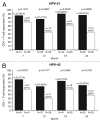
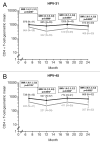
Protection against oncogenic non-vaccine types (cross-protection) offered by human papillomavirus (HPV) vaccines may provide a significant medical benefit. Available clinical efficacy data suggest the two licensed vaccines (HPV-16/18 vaccine, GlaxoSmithKline Biologicals (GSK), and HPV-6/11/16/18 vaccine, Merck & Co., Inc.) differ in terms of protection against oncogenic non-vaccine HPV types -31/45. The immune responses induced by the two vaccines against these two non-vaccine HPV types (cross-reactivity) was compared in an observer-blind study up to Month 24 (18 mo post-vaccination), in women HPV DNA-negative and seronegative prior to vaccination for the HPV type analyzed (HPV-010 [NCT00423046]). Geometric mean antibody titers (GMTs) measured by pseudovirion-based neutralization assay (PBNA) and enzyme-linked immunosorbent assay (ELISA) were similar between vaccines for HPV-31/45. Seropositivity rates for HPV-31 were also similar between vaccines; however, there was a trend for higher seropositivity with the HPV-16/18 vaccine (13.0-16.7%) versus the HPV-6/11/16/18 vaccine (0.0-5.0%) for HPV-45 with PBNA, but not ELISA. HPV-31/45 cross-reactive memory B-cell responses were comparable between vaccines. Circulating antigen-specific CD4+ T-cell frequencies were higher for the HPV-16/18 vaccine than the HPV-6/11/16/18 vaccine (HPV-31 [geometric mean ratio [GMR] =2.0; p=0.0002] and HPV-45 [GMR=2.6; p=0.0092]), as were the proportion of T-cell responders (HPV-31, p=0.0009; HPV-45, p=0.0793). In conclusion, immune response to oncogenic non-vaccine HPV types -31/45 was generally similar for both vaccines with the exception of T-cell response which was higher with the HPV-16/18 vaccine. Considering the differences in cross-protective efficacy between the two vaccines, the results might provide insights into the underlying mechanism(s) of protection.
Figures
Similar articles
-
Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study.Hum Vaccin Immunother. 2014;10(12):3455-65. doi: 10.4161/hv.36117. Hum Vaccin Immunother. 2014. PMID: 25483700 Free PMC article. Clinical Trial.
-
Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years.Hum Vaccin. 2011 Dec;7(12):1343-58. doi: 10.4161/hv.7.12.18281. Epub 2011 Dec 1. Hum Vaccin. 2011. PMID: 22048173 Free PMC article. Clinical Trial.
-
Comparison of the immunogenicity of Cervarix® and Gardasil® human papillomavirus vaccines for oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults.Hum Vaccin Immunother. 2014;10(5):1147-54. doi: 10.4161/hv.27925. Epub 2014 Feb 19. Hum Vaccin Immunother. 2014. PMID: 24553190 Free PMC article. Clinical Trial.
-
Expanded strain coverage for a highly successful public health tool: Prophylactic 9-valent human papillomavirus vaccine.Hum Vaccin Immunother. 2017 Oct 3;13(10):2280-2291. doi: 10.1080/21645515.2017.1346755. Hum Vaccin Immunother. 2017. PMID: 28699820 Free PMC article. Review.
-
Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis.Lancet Infect Dis. 2012 Oct;12(10):781-9. doi: 10.1016/S1473-3099(12)70187-1. Epub 2012 Aug 22. Lancet Infect Dis. 2012. PMID: 22920953 Review.
Cited by
-
Substantial Decline in Prevalence of Vaccine-Type and Nonvaccine-Type Human Papillomavirus (HPV) in Vaccinated and Unvaccinated Girls 5 Years After Implementing HPV Vaccine in Norway.J Infect Dis. 2018 Nov 5;218(12):1900-1910. doi: 10.1093/infdis/jiy432. J Infect Dis. 2018. PMID: 30010913 Free PMC article.
-
Selective Persistence of HPV Cross-Neutralising Antibodies following Reduced-Dose HPV Vaccine Schedules.Vaccines (Basel). 2019 Nov 28;7(4):200. doi: 10.3390/vaccines7040200. Vaccines (Basel). 2019. PMID: 31795211 Free PMC article.
-
Long-term Cross-reactivity Against Nonvaccine Human Papillomavirus Types 31 and 45 After 2- or 3-Dose Schedules of the AS04-Adjuvanted Human HPV-16/18 Vaccine.J Infect Dis. 2019 May 5;219(11):1799-1803. doi: 10.1093/infdis/jiy743. J Infect Dis. 2019. PMID: 30715452 Free PMC article. Clinical Trial.
-
Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study.Hum Vaccin Immunother. 2014;10(12):3455-65. doi: 10.4161/hv.36117. Hum Vaccin Immunother. 2014. PMID: 25483700 Free PMC article. Clinical Trial.
-
Understanding and learning from the success of prophylactic human papillomavirus vaccines.Nat Rev Microbiol. 2012 Oct;10(10):681-92. doi: 10.1038/nrmicro2872. Epub 2012 Sep 10. Nat Rev Microbiol. 2012. PMID: 22961341 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
