Modulation of GABA transport by adenosine A1R-A2AR heteromers, which are coupled to both Gs- and G(i/o)-proteins
- PMID: 22049406
- PMCID: PMC6623011
- DOI: 10.1523/JNEUROSCI.2526-11.2011
Modulation of GABA transport by adenosine A1R-A2AR heteromers, which are coupled to both Gs- and G(i/o)-proteins
Retraction in
-
Retraction: Cristóvão-Ferreira et al., Modulation of GABA transport by adenosine A1R-A2AR heteromers, which are coupled to both Gs- and G(i/o)-proteins.J Neurosci. 2013 Jan 16;33(3):1292. doi: 10.1523/JNEUROSCI.5705-12.2013. J Neurosci. 2013. PMID: 23325265 Free PMC article. No abstract available.
Abstract
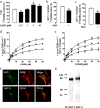
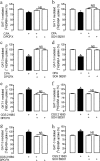
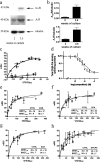
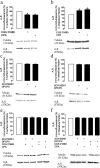
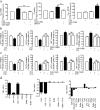
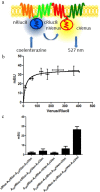
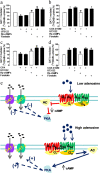
Astrocytes play a key role in modulating synaptic transmission by controlling the available extracellular GABA via the GAT-1 and GAT-3 GABA transporters (GATs). Using primary cultures of rat astrocytes, we show here that an additional level of regulation of GABA uptake occurs via modulation of the GATs by the adenosine A(1) (A(1)R) and A(2A) (A(2A)R) receptors. This regulation occurs through a complex of heterotetramers (two interacting homodimers) of A(1)R-A(2A)R that signal via two different G-proteins, G(s) and G(i/o), and either enhances (A(2A)R) or inhibits (A(1)R) GABA uptake. These results provide novel mechanistic insight into how G-protein-coupled receptor heteromers signal. Furthermore, we uncover a previously unknown mechanism in which adenosine, in a concentration-dependent manner, acts via a heterocomplex of adenosine receptors in astrocytes to significantly contribute to neurotransmission at the tripartite (neuron-glia-neuron) synapse.
Figures

Similar articles
-
A1R-A2AR heteromers coupled to Gs and G i/0 proteins modulate GABA transport into astrocytes.Purinergic Signal. 2013 Sep;9(3):433-49. doi: 10.1007/s11302-013-9364-5. Epub 2013 May 10. Purinergic Signal. 2013. PMID: 23657626 Free PMC article.
-
Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes.J Biol Chem. 2011 Nov 25;286(47):40464-76. doi: 10.1074/jbc.M111.232009. Epub 2011 Oct 3. J Biol Chem. 2011. PMID: 21969376 Free PMC article.
-
Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain.BMC Biol. 2018 Feb 28;16(1):24. doi: 10.1186/s12915-018-0491-x. BMC Biol. 2018. PMID: 29486745 Free PMC article.
-
P2Y1 receptor inhibits GABA transport through a calcium signalling-dependent mechanism in rat cortical astrocytes.Glia. 2014 Aug;62(8):1211-26. doi: 10.1002/glia.22673. Epub 2014 Apr 15. Glia. 2014. PMID: 24733747
-
Astrocytic GABA Transporters: Pharmacological Properties and _targets for Antiepileptic Drugs.Adv Neurobiol. 2017;16:283-296. doi: 10.1007/978-3-319-55769-4_14. Adv Neurobiol. 2017. PMID: 28828616 Review.
Cited by
-
Correction to: A1R-A2AR heteromers coupled to Gs and Gi/0 proteins modulate GABA transport into astrocytes.Purinergic Signal. 2024 Jun;20(3):315-316. doi: 10.1007/s11302-024-10012-3. Purinergic Signal. 2024. PMID: 38671309 Free PMC article. No abstract available.
-
Adenosine A1 receptors heterodimerize with β1- and β2-adrenergic receptors creating novel receptor complexes with altered G protein coupling and signaling.Cell Signal. 2013 Apr;25(4):736-42. doi: 10.1016/j.cellsig.2012.12.022. Epub 2013 Jan 3. Cell Signal. 2013. PMID: 23291003 Free PMC article.
-
Adenosine heteroreceptor complexes in the basal ganglia are implicated in Parkinson's disease and its treatment.J Neural Transm (Vienna). 2019 Apr;126(4):455-471. doi: 10.1007/s00702-019-01969-2. Epub 2019 Jan 14. J Neural Transm (Vienna). 2019. PMID: 30637481 Free PMC article. Review.
-
Fractalkine (CX3CL1) enhances hippocampal N-methyl-D-aspartate receptor (NMDAR) function via D-serine and adenosine receptor type A2 (A2AR) activity.J Neuroinflammation. 2013 Aug 27;10:108. doi: 10.1186/1742-2094-10-108. J Neuroinflammation. 2013. PMID: 23981568 Free PMC article.
-
Glutamate Transporters in Hippocampal LTD/LTP: Not Just Prevention of Excitotoxicity.Front Cell Neurosci. 2019 Aug 6;13:357. doi: 10.3389/fncel.2019.00357. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31447647 Free PMC article. Review.
References
-
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. - PubMed
-
- Awad JA, Johnson RA, Jakobs KH, Schultz G. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J Biol Chem. 1983;258:2960–2965. - PubMed
-
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. - PubMed
-
- Bokoch GM, Gilman AG. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984;39:301–308. - PubMed
-
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, Ferré S, Lluis C, Bouvier M, Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
