Prorenin receptor is essential for normal podocyte structure and function
- PMID: 22052048
- PMCID: PMC3279932
- DOI: 10.1681/ASN.2011020202
Prorenin receptor is essential for normal podocyte structure and function
Abstract
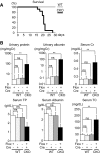
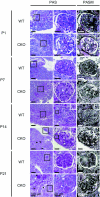
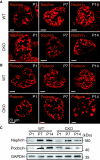
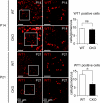
The prorenin receptor is an accessory subunit of the vacuolar H(+)-ATPase, suggesting that it has fundamental functions beyond activation of the local renin-angiotensin system. Podocytes express the prorenin receptor, but its function in these cells is unknown. Here, podocyte-specific, conditional, prorenin receptor-knockout mice died of kidney failure and severe proteinuria within 4 weeks of birth. The podocytes of these mice exhibited foot process effacement with reduced and altered localization of the slit-diaphragm proteins nephrin and podocin. Furthermore, the podocytes contained numerous autophagic vacuoles, confirmed by enhanced accumulation of microtubule-associated protein 1 light chain 3-positive intracellular vesicles. Ablation of the prorenin receptor selectively suppressed expression of the V(0) c-subunit of the vacuolar H(+)-ATPase in podocytes, resulting in deacidification of intracellular vesicles. In conclusion, the prorenin receptor is important for the maintenance of normal podocyte structure and function.
Figures


Comment in
-
The prorenin receptor: what's in a name.J Am Soc Nephrol. 2011 Dec;22(12):2141-3. doi: 10.1681/ASN.2011100981. Epub 2011 Nov 3. J Am Soc Nephrol. 2011. PMID: 22052050 No abstract available.
-
Basic research: Prorenin receptor is needed for podocyte function.Nat Rev Nephrol. 2011 Nov 29;8(1):2. doi: 10.1038/nrneph.2011.181. Nat Rev Nephrol. 2011. PMID: 22124171 No abstract available.
Similar articles
-
Prorenin receptor is essential for podocyte autophagy and survival.J Am Soc Nephrol. 2011 Dec;22(12):2193-202. doi: 10.1681/ASN.2011020200. Epub 2011 Oct 27. J Am Soc Nephrol. 2011. PMID: 22034640 Free PMC article.
-
Prorenin receptor is critical for nephron progenitors.Dev Biol. 2016 Jan 15;409(2):382-91. doi: 10.1016/j.ydbio.2015.11.024. Epub 2015 Dec 3. Dev Biol. 2016. PMID: 26658320 Free PMC article.
-
The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes.Circ Res. 2010 Jul 9;107(1):30-4. doi: 10.1161/CIRCRESAHA.110.224667. Epub 2010 Jun 22. Circ Res. 2010. PMID: 20570919
-
The renin-angiotensin-aldosterone system in podocytes.Semin Nephrol. 2012 Jul;32(4):377-84. doi: 10.1016/j.semnephrol.2012.06.009. Semin Nephrol. 2012. PMID: 22958492 Review.
-
Podocytes as a _target of prorenin in diabetes.Curr Diabetes Rev. 2011 Jan;7(1):17-21. doi: 10.2174/157339911794273955. Curr Diabetes Rev. 2011. PMID: 21067509 Review.
Cited by
-
Vacuolar ATPase in phagosome-lysosome fusion.J Biol Chem. 2015 May 29;290(22):14166-80. doi: 10.1074/jbc.M114.628891. Epub 2015 Apr 22. J Biol Chem. 2015. PMID: 25903133 Free PMC article.
-
Autophagy in Immune-Related Renal Disease.J Immunol Res. 2019 Nov 7;2019:5071687. doi: 10.1155/2019/5071687. eCollection 2019. J Immunol Res. 2019. PMID: 31815154 Free PMC article. Review.
-
Stromal prorenin receptor is critical for normal kidney development.Am J Physiol Regul Integr Comp Physiol. 2019 May 1;316(5):R640-R650. doi: 10.1152/ajpregu.00320.2018. Epub 2019 Apr 3. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 30943054 Free PMC article.
-
pHluorin-BACE1-mCherry Acts as a Reporter for the Intracellular Distribution of Active BACE1 In Vitro and In Vivo.Cells. 2019 May 17;8(5):474. doi: 10.3390/cells8050474. Cells. 2019. PMID: 31108937 Free PMC article.
-
Autophagy and Renal Fibrosis.Aging Dis. 2022 Jun 1;13(3):712-731. doi: 10.14336/AD.2021.1027. eCollection 2022 Jun. Aging Dis. 2022. PMID: 35656109 Free PMC article. Review.
References
-
- Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T: Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004 - PMC - PubMed
-
- Ichihara A, Sakoda M, Kurauchi-Mito A, Nishiyama A, Itoh H: Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens 2: 332–340, 2008 - PubMed
-
- Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T: Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 17: 1950–1961, 2006 - PubMed
-
- Takahashi H, Ichihara A, Kaneshiro Y, Inomata K, Sakoda M, Takemitsu T, Nishiyama A, Itoh H: Regression of nephropathy developed in diabetes by (pro)renin receptor blockade. J Am Soc Nephrol 18: 2054–2061, 2007 - PubMed
-
- Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Nakagawa T, Nishiyama A, Kawachi H, Shimizu F, Inagami T: Contribution of nonproteolytically activated prorenin in glomeruli to hypertensive renal damage. J Am Soc Nephrol 17: 2495–2503, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

