RpkA, a highly conserved GPCR with a lipid kinase domain, has a role in phagocytosis and anti-bacterial defense
- PMID: 22073313
- PMCID: PMC3206951
- DOI: 10.1371/journal.pone.0027311
RpkA, a highly conserved GPCR with a lipid kinase domain, has a role in phagocytosis and anti-bacterial defense
Abstract
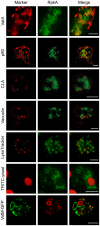
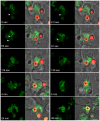
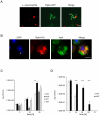
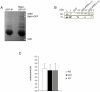
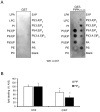
RpkA (Receptor phosphatidylinositol kinase A) is an unusual seven-helix transmembrane protein of Dictyostelium discoideum with a G protein coupled receptor (GPCR) signature and a C-terminal lipid kinase domain (GPCR-PIPK) predicted as a phosphatidylinositol-4-phosphate 5-kinase. RpkA-homologs are present in all so far sequenced Dictyostelidae as well as in several other lower eukaryotes like the oomycete Phytophthora, and in the Legionella host Acanthamoeba castellani. Here we show by immunofluorescence that RpkA localizes to endosomal membranes and is specifically recruited to phagosomes. RpkA interacts with the phagosomal protein complex V-ATPase as proteins of this complex co-precipitate with RpkA-GFP as well as with the GST-tagged PIPK domain of RpkA. Loss of RpkA leads to a defect in phagocytosis as measured by yeast particle uptake. The uptake of the pathogenic bacterium Legionella pneumophila was however unaltered whereas its intra-cellular replication was significantly enhanced in rpkA(-). The difference between wild type and rpkA(-) was even more prominent when L. hackeliae was used. When we investigated the reason for the enhanced susceptibility for L. pneumophila of rpkA(-) we could not detect a difference in endosomal pH but rpkA(-) showed depletion of phosphoinositides (PIP and PIP(2)) when we compared metabolically labeled phosphoinositides from wild type and rpkA(-). Furthermore rpkA(-) exhibited reduced nitrogen starvation tolerance, an indicator for a reduced autophagy rate. Our results indicate that RpkA is a component of the defense system of D. discoideum as well as other lower eukaryotes.
Conflict of interest statement
Figures
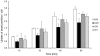
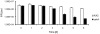
Similar articles
-
A G protein-coupled receptor with a lipid kinase domain is involved in cell-density sensing.Curr Biol. 2007 May 15;17(10):892-7. doi: 10.1016/j.cub.2007.04.029. Epub 2007 May 3. Curr Biol. 2007. PMID: 17481898
-
Macroautophagy is dispensable for intracellular replication of Legionella pneumophila in Dictyostelium discoideum.Mol Microbiol. 2004 Jan;51(1):63-72. doi: 10.1046/j.1365-2958.2003.03826.x. Mol Microbiol. 2004. PMID: 14651611
-
Icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila.Mol Microbiol. 2001 Nov;42(3):603-17. doi: 10.1046/j.1365-2958.2001.02645.x. Mol Microbiol. 2001. PMID: 11722729
-
Acanthamoeba and Dictyostelium as Cellular Models for Legionella Infection.Front Cell Infect Microbiol. 2018 Mar 2;8:61. doi: 10.3389/fcimb.2018.00061. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29552544 Free PMC article. Review.
-
Modulation of host cell function by Legionella pneumophila type IV effectors.Annu Rev Cell Dev Biol. 2010;26:261-83. doi: 10.1146/annurev-cellbio-100109-104034. Annu Rev Cell Dev Biol. 2010. PMID: 20929312 Review.
Cited by
-
Dictyostelium Dynamin Superfamily GTPases Implicated in Vesicle Trafficking and Host-Pathogen Interactions.Front Cell Dev Biol. 2021 Oct 13;9:731964. doi: 10.3389/fcell.2021.731964. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34746129 Free PMC article. Review.
-
Whole Organism Model to Study Molecular Mechanisms of Differentiation and Dedifferentiation.Biology (Basel). 2020 Apr 17;9(4):79. doi: 10.3390/biology9040079. Biology (Basel). 2020. PMID: 32316619 Free PMC article.
-
Interactome and F-Actin Interaction Analysis of Dictyostelium discoideum Coronin A.Int J Mol Sci. 2020 Feb 21;21(4):1469. doi: 10.3390/ijms21041469. Int J Mol Sci. 2020. PMID: 32098122 Free PMC article.
-
The Dictyostelium discoideum RACK1 orthologue has roles in growth and development.Cell Commun Signal. 2014 Jun 15;12:37. doi: 10.1186/1478-811X-12-37. Cell Commun Signal. 2014. PMID: 24930026 Free PMC article.
-
Peroxisome proliferator-activated receptor γ (PPARγ): A master gatekeeper in CNS injury and repair.Prog Neurobiol. 2018 Apr-May;163-164:27-58. doi: 10.1016/j.pneurobio.2017.10.002. Epub 2017 Oct 12. Prog Neurobiol. 2018. PMID: 29032144 Free PMC article. Review.
References
-
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
-
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. - PubMed
-
- Yates RM, Russell DG. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity. 2005;23:409–417. - PubMed
-
- Cornillon S, Pech E, Benghezal M, Ravanel K, Gaynor E, et al. Phg1p is a nine-transmembrane protein superfamily member involved in Dictyostelium adhesion and phagocytosis. The Journal of biological chemistry. 2000;275:34287–34292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

