Mutations causing syndromic autism define an axis of synaptic pathophysiology
- PMID: 22113615
- PMCID: PMC3228874
- DOI: 10.1038/nature10658
Mutations causing syndromic autism define an axis of synaptic pathophysiology
Abstract
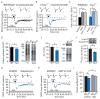
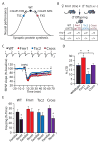
Tuberous sclerosis complex and fragile X syndrome are genetic diseases characterized by intellectual disability and autism. Because both syndromes are caused by mutations in genes that regulate protein synthesis in neurons, it has been hypothesized that excessive protein synthesis is one core pathophysiological mechanism of intellectual disability and autism. Using electrophysiological and biochemical assays of neuronal protein synthesis in the hippocampus of Tsc2(+/-) and Fmr1(-/y) mice, here we show that synaptic dysfunction caused by these mutations actually falls at opposite ends of a physiological spectrum. Synaptic, biochemical and cognitive defects in these mutants are corrected by treatments that modulate metabotropic glutamate receptor 5 in opposite directions, and deficits in the mutants disappear when the mice are bred to carry both mutations. Thus, normal synaptic plasticity and cognition occur within an optimal range of metabotropic glutamate-receptor-mediated protein synthesis, and deviations in either direction can lead to shared behavioural impairments.
Figures


Comment in
-
Neurodevelopmental disorders: a fragile synaptic balance.Nat Rev Neurosci. 2012 Jan;13(1):3. doi: 10.1038/nrn3163. Nat Rev Neurosci. 2012. PMID: 22295280 No abstract available.
Similar articles
-
Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome.Nat Neurosci. 2012 Jan 22;15(3):431-40, S1. doi: 10.1038/nn.3033. Nat Neurosci. 2012. PMID: 22267161 Free PMC article.
-
β-Arrestin2 Couples Metabotropic Glutamate Receptor 5 to Neuronal Protein Synthesis and Is a Potential _target to Treat Fragile X.Cell Rep. 2017 Mar 21;18(12):2807-2814. doi: 10.1016/j.celrep.2017.02.075. Cell Rep. 2017. PMID: 28329674 Free PMC article.
-
Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11591-6. doi: 10.1073/pnas.1002262107. Epub 2010 Jun 7. Proc Natl Acad Sci U S A. 2010. PMID: 20534533 Free PMC article.
-
Toward fulfilling the promise of molecular medicine in fragile X syndrome.Annu Rev Med. 2011;62:411-29. doi: 10.1146/annurev-med-061109-134644. Annu Rev Med. 2011. PMID: 21090964 Free PMC article. Review.
-
Dysregulation of group-I metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with Intellectual Disability and Autism.Neurosci Biobehav Rev. 2014 Oct;46 Pt 2(Pt 2):228-41. doi: 10.1016/j.neubiorev.2014.02.003. Epub 2014 Feb 15. Neurosci Biobehav Rev. 2014. PMID: 24548786 Free PMC article. Review.
Cited by
-
Fragile X mental retardation protein: from autism to neurodegenerative disease.Front Cell Neurosci. 2015 Feb 12;9:43. doi: 10.3389/fncel.2015.00043. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25729352 Free PMC article. No abstract available.
-
Neural connectivity abnormalities in autism: insights from the Tuberous Sclerosis model.BMC Med. 2013 Feb 27;11:55. doi: 10.1186/1741-7015-11-55. BMC Med. 2013. PMID: 23445933 Free PMC article.
-
Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex.Nat Commun. 2012;3:1292. doi: 10.1038/ncomms2295. Nat Commun. 2012. PMID: 23250422 Free PMC article.
-
Metabolic dynamics in astrocytes and microglia during post-natal development and their implications for autism spectrum disorders.Front Cell Neurosci. 2024 Feb 14;18:1354259. doi: 10.3389/fncel.2024.1354259. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38419654 Free PMC article. Review.
-
_targeted treatment trials for tuberous sclerosis and autism: no longer a dream.Curr Opin Neurobiol. 2012 Oct;22(5):895-901. doi: 10.1016/j.conb.2012.04.008. Epub 2012 May 4. Curr Opin Neurobiol. 2012. PMID: 22560338 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

