IAPs regulate the plasticity of cell migration by directly _targeting Rac1 for degradation
- PMID: 22117219
- PMCID: PMC3252583
- DOI: 10.1038/emboj.2011.423
IAPs regulate the plasticity of cell migration by directly _targeting Rac1 for degradation
Abstract
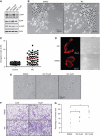
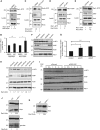
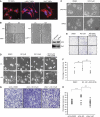
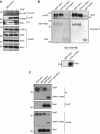
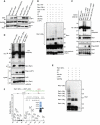
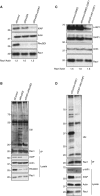
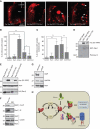
Inhibitors of apoptosis proteins (IAPs) are a highly conserved class of multifunctional proteins. Rac1 is a well-studied Rho GTPase that controls numerous basic cellular processes. While the regulation of nucleotide binding to Rac1 is well understood, the molecular mechanisms controlling Rac1 degradation are not known. Here, we demonstrate X-linked IAP (XIAP) and cellular IAP1 (c-IAP1) directly bind to Rac1 in a nucleotide-independent manner to promote its polyubiquitination at Lys147 and proteasomal degradation. These IAPs are also required for degradation of Rac1 upon CNF1 toxin treatment or RhoGDI depletion. Consistently, downregulation of XIAP or c-IAP1 by various strategies led to an increase in Rac1 protein levels in primary and tumour cells, leading to an elongated morphology and enhanced cell migration. Further, XIAP counteracts Rac1-dependent cellular polarization in the developing zebrafish hindbrain and promotes the delamination of neurons from the normal tissue architecture. These observations unveil an evolutionarily conserved role of IAPs in controlling Rac1 stability thereby regulating the plasticity of cell migration and morphogenesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment in
-
Ubiquitin-mediated regulation of RhoGTPase signalling: IAPs and HACE1 enter the fray.EMBO J. 2012 Jan 4;31(1):1-2. doi: 10.1038/emboj.2011.452. Epub 2012 Jan 4. EMBO J. 2012. PMID: 22215447 Free PMC article.
Similar articles
-
IAPs as E3 ligases of Rac1: shaping the move.Small GTPases. 2012 Apr-Jun;3(2):131-6. doi: 10.4161/sgtp.19988. Small GTPases. 2012. PMID: 22790203 Free PMC article.
-
Ubiquitination of Rac1 by inhibitors of apoptosis (IAPs).Methods Mol Biol. 2014;1120:43-54. doi: 10.1007/978-1-62703-791-4_4. Methods Mol Biol. 2014. PMID: 24470018
-
IAPs on the move: role of inhibitors of apoptosis proteins in cell migration.Cell Death Dis. 2013 Sep 5;4(9):e784. doi: 10.1038/cddis.2013.311. Cell Death Dis. 2013. PMID: 24008728 Free PMC article. Review.
-
Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2(1).Biochem J. 2009 Jan 1;417(1):149-60. doi: 10.1042/BJ20081885. Biochem J. 2009. PMID: 18939944
-
IAP family of cell death and signaling regulators.Methods Enzymol. 2014;545:35-65. doi: 10.1016/B978-0-12-801430-1.00002-0. Methods Enzymol. 2014. PMID: 25065885 Review.
Cited by
-
Identification of non-canonical NF-κB signaling as a critical mediator of Smac mimetic-stimulated migration and invasion of glioblastoma cells.Cell Death Dis. 2013 Mar 28;4(3):e564. doi: 10.1038/cddis.2013.70. Cell Death Dis. 2013. PMID: 23538445 Free PMC article.
-
The E3 ubiquitin ligase Hace1 is required for early embryonic development in Xenopus laevis.BMC Dev Biol. 2016 Sep 21;16(1):31. doi: 10.1186/s12861-016-0132-y. BMC Dev Biol. 2016. PMID: 27653971 Free PMC article.
-
Dysregulation of Rho GTPases in Human Cancers.Cancers (Basel). 2020 May 7;12(5):1179. doi: 10.3390/cancers12051179. Cancers (Basel). 2020. PMID: 32392742 Free PMC article. Review.
-
RABV induces biphasic actin cytoskeletal rearrangement through Rac1 activity modulation.J Virol. 2024 Jul 23;98(7):e0060624. doi: 10.1128/jvi.00606-24. Epub 2024 May 29. J Virol. 2024. PMID: 38809020 Free PMC article.
-
Apoptosis - Fueling the oncogenic fire.FEBS J. 2021 Aug;288(15):4445-4463. doi: 10.1111/febs.15624. Epub 2020 Nov 25. FEBS J. 2021. PMID: 33179432 Free PMC article. Review.
References
-
- Bertrand MJ, Doiron K, Labbe K, Korneluk RG, Barker PA, Saleh M (2009) Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30: 789–801 - PubMed
-
- Blankenship JW, Varfolomeev E, Goncharov T, Fedorova AV, Kirkpatrick DS, Izrael-Tomasevic A, Phu L, Arnott D, Aghajan M, Zobel K, Bazan JF, Fairbrother WJ, Deshayes K, Vucic D (2008) Ubiquitin binding modulates IAP antagonist stimulated proteasomal degradation of c IAP1 and c IAP2. Biochem J 417: 1–3 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

