The role of sirtuin 2 activation by nicotinamide phosphoribosyltransferase in the aberrant proliferation and survival of myeloid leukemia cells
- PMID: 22207684
- PMCID: PMC3347680
- DOI: 10.3324/haematol.2011.055236
The role of sirtuin 2 activation by nicotinamide phosphoribosyltransferase in the aberrant proliferation and survival of myeloid leukemia cells
Abstract
Background: Inhibitors of nicotinamide phosphoribosyltransferase have recently been validated as therapeutic _targets in leukemia, but the mechanism of leukemogenic transformation downstream of this enzyme is unclear.
Design and methods: Here, we evaluated whether nicotinamide phosphoribosyltransferase's effects on aberrant proliferation and survival of myeloid leukemic cells are dependent on sirtuin and delineated the downstream signaling pathways operating during this process.
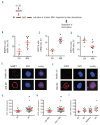
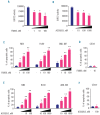
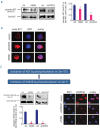
Results: We identified significant upregulation of sirtuin 2 and nicotinamide phosphoribosyltransferase levels in primary acute myeloid leukemia blasts compared to in hematopoietic progenitor cells from healthy individuals. Importantly, specific inhibition of nicotinamide phosphoribosyltransferase or sirtuin 2 significantly reduced proliferation and induced apoptosis in human acute myeloid leukemia cell lines and primary blasts. Intriguingly, we found that protein kinase B/AKT could be deacetylated by nicotinamide phosphoribosyltransferase and sirtuin 2. The anti-leukemic effects of the inhibition of nicotinamide phosphoribosyltransferase or sirtuin 2 were accompanied by acetylation of protein kinase B/AKT with subsequent inhibition by dephosphorylation. This leads to activation of glycogen synthase kinase-3 β via diminished phosphorylation and, ultimately, inactivation of β-catenin by phosphorylation.
Conclusions: Our results provide strong evidence that nicotinamide phosphoribosyltransferase and sirtuin 2 participate in the aberrant proliferation and survival of leukemic cells, and suggest that the protein kinase B/AKT/ glycogen synthase kinase-3 β/β-catenin pathway is a _target for inhibition of nicotinamide phosphoribosyltransferase or sirtuin 2 and, thereby, leukemia cell proliferation.
Figures
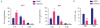

Similar articles
-
SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3β/β-catenin signaling.Hepatology. 2013 Jun;57(6):2287-98. doi: 10.1002/hep.26278. Epub 2013 Apr 26. Hepatology. 2013. PMID: 23348706
-
Sirtuin 2 Isoform 1 Enhances Hepatitis B Virus RNA Transcription and DNA Synthesis through the AKT/GSK-3β/β-Catenin Signaling Pathway.J Virol. 2018 Oct 12;92(21):e00955-18. doi: 10.1128/JVI.00955-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30111572 Free PMC article.
-
Anti-proliferation effect of APO866 on C6 glioblastoma cells by inhibiting nicotinamide phosphoribosyltransferase.Eur J Pharmacol. 2012 Jan 15;674(2-3):163-70. doi: 10.1016/j.ejphar.2011.11.017. Epub 2011 Nov 19. Eur J Pharmacol. 2012. PMID: 22119381
-
Nicotinamide phosphoribosyltransferase: a potent therapeutic _target in non-small cell lung cancer with epidermal growth factor receptor-gene mutation.J Thorac Oncol. 2012 Jan;7(1):49-56. doi: 10.1097/JTO.0b013e318233d686. J Thorac Oncol. 2012. PMID: 22089115
-
Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation.Cancer Res. 2008 May 15;68(10):3733-42. doi: 10.1158/0008-5472.CAN-07-2509. Cancer Res. 2008. PMID: 18483256 Free PMC article.
Cited by
-
Sirtuin catalysis and regulation.J Biol Chem. 2012 Dec 14;287(51):42419-27. doi: 10.1074/jbc.R112.378877. Epub 2012 Oct 18. J Biol Chem. 2012. PMID: 23086947 Free PMC article. Review.
-
The SIRT2 Deacetylase Stabilizes Slug to Control Malignancy of Basal-like Breast Cancer.Cell Rep. 2016 Oct 25;17(5):1302-1317. doi: 10.1016/j.celrep.2016.10.006. Cell Rep. 2016. PMID: 27783945 Free PMC article.
-
Emerging role of sirtuins on tumorigenesis: possible link between aging and cancer.BMB Rep. 2013 Sep;46(9):429-38. doi: 10.5483/bmbrep.2013.46.9.180. BMB Rep. 2013. PMID: 24064057 Free PMC article. Review.
-
Sirtuin 2 knockdown inhibits cell proliferation and RAS/ERK signaling, and promotes cell apoptosis and cell cycle arrest in multiple myeloma.Mol Med Rep. 2021 Nov;24(5):760. doi: 10.3892/mmr.2021.12400. Epub 2021 Sep 3. Mol Med Rep. 2021. PMID: 34476507 Free PMC article.
-
SIRT2 is an unfavorable prognostic biomarker in patients with acute myeloid leukemia.Sci Rep. 2016 Jun 13;6:27694. doi: 10.1038/srep27694. Sci Rep. 2016. PMID: 27291931 Free PMC article.
References
-
- Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15(2):151–8. - PubMed
-
- Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV, et al. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood. 2009;113(14):3276–86. - PubMed
-
- Guarente L. Sirtuins, aging, and medicine. N Eng J Med. 2011;364(23):2235–2244. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

