ald of Mycobacterium tuberculosis encodes both the alanine dehydrogenase and the putative glycine dehydrogenase
- PMID: 22210765
- PMCID: PMC3294785
- DOI: 10.1128/JB.05914-11
ald of Mycobacterium tuberculosis encodes both the alanine dehydrogenase and the putative glycine dehydrogenase
Abstract
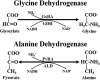
The putative glycine dehydrogenase of Mycobacterium tuberculosis catalyzes the reductive amination of glyoxylate to glycine but not the reverse reaction. The enzyme was purified and identified as the previously characterized alanine dehydrogenase. The Ald enzyme was expressed in Escherichia coli and had both pyruvate and glyoxylate aminating activities. The gene, ald, was inactivated in M. tuberculosis, which resulted in the loss of all activities. Both enzyme activities were found associated with the cell and were not detected in the extracellular filtrate. By using an anti-Ald antibody, the protein was localized to the cell membrane, with a smaller fraction in the cytosol. None was detected in the extracellular medium. The ald knockout strain grew without alanine or glycine and was able to utilize glycine but not alanine as a nitrogen source. Transcription of ald was induced when alanine was the sole nitrogen source, and higher levels of Ald enzyme were measured. Ald is proposed to have several functions, including ammonium incorporation and alanine breakdown.
Figures

Similar articles
-
Regulation Mechanism of the ald Gene Encoding Alanine Dehydrogenase in Mycobacterium smegmatis and Mycobacterium tuberculosis by the Lrp/AsnC Family Regulator AldR.J Bacteriol. 2015 Oct;197(19):3142-53. doi: 10.1128/JB.00453-15. Epub 2015 Jul 20. J Bacteriol. 2015. PMID: 26195594 Free PMC article.
-
Alanine dehydrogenases in mycobacteria.J Microbiol. 2019 Feb;57(2):81-92. doi: 10.1007/s12275-019-8543-7. Epub 2019 Jan 31. J Microbiol. 2019. PMID: 30706339 Review.
-
Role of Alanine Dehydrogenase of Mycobacterium tuberculosis during Recovery from Hypoxic Nonreplicating Persistence.PLoS One. 2016 May 20;11(5):e0155522. doi: 10.1371/journal.pone.0155522. eCollection 2016. PLoS One. 2016. PMID: 27203084 Free PMC article.
-
Mycobacterium smegmatis L-alanine dehydrogenase (Ald) is required for proficient utilization of alanine as a sole nitrogen source and sustained anaerobic growth.J Bacteriol. 2002 Sep;184(18):5001-10. doi: 10.1128/JB.184.18.5001-5010.2002. J Bacteriol. 2002. PMID: 12193615 Free PMC article.
-
Alanine dehydrogenase and its applications - A review.Crit Rev Biotechnol. 2019 Aug;39(5):648-664. doi: 10.1080/07388551.2019.1594153. Epub 2019 Apr 24. Crit Rev Biotechnol. 2019. PMID: 31018703 Review.
Cited by
-
Regulation Mechanism of the ald Gene Encoding Alanine Dehydrogenase in Mycobacterium smegmatis and Mycobacterium tuberculosis by the Lrp/AsnC Family Regulator AldR.J Bacteriol. 2015 Oct;197(19):3142-53. doi: 10.1128/JB.00453-15. Epub 2015 Jul 20. J Bacteriol. 2015. PMID: 26195594 Free PMC article.
-
Systems-based approaches to probing metabolic variation within the Mycobacterium tuberculosis complex.PLoS One. 2013 Sep 17;8(9):e75913. doi: 10.1371/journal.pone.0075913. eCollection 2013. PLoS One. 2013. PMID: 24098743 Free PMC article.
-
Mycobacterium tuberculosis suppresses host antimicrobial peptides by dehydrogenating L-alanine.Nat Commun. 2024 May 17;15(1):4216. doi: 10.1038/s41467-024-48588-4. Nat Commun. 2024. PMID: 38760394 Free PMC article.
-
Alanine dehydrogenases in mycobacteria.J Microbiol. 2019 Feb;57(2):81-92. doi: 10.1007/s12275-019-8543-7. Epub 2019 Jan 31. J Microbiol. 2019. PMID: 30706339 Review.
-
Combined Methylome, Transcriptome and Proteome Analyses Document Rapid Acclimatization of a Bacterium to Environmental Changes.Front Microbiol. 2020 Sep 15;11:544785. doi: 10.3389/fmicb.2020.544785. eCollection 2020. Front Microbiol. 2020. PMID: 33042055 Free PMC article.
References
-
- Agren D, et al. 2008. Three-dimensional structures of apo- and holo-L-alanine dehydrogenase from Mycobacterium tuberculosis reveal conformational changes upon coenzyme binding. J. Mol. Biol. 377:1161–1173 - PubMed
-
- Awasthy D, et al. 2009. Inactivation of the ilvB1 gene in Mycobacterium tuberculosis leads to branched-chain amino acid auxotrophy and attenuation of virulence in mice. Microbiology 155:2978–2987 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

