Nuclear localization of lymphocyte-specific protein tyrosine kinase (Lck) and its role in regulating LIM domain only 2 (Lmo2) gene
- PMID: 22222369
- PMCID: PMC3264771
- DOI: 10.1016/j.bbrc.2011.12.095
Nuclear localization of lymphocyte-specific protein tyrosine kinase (Lck) and its role in regulating LIM domain only 2 (Lmo2) gene
Abstract
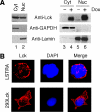
LIM domain only protein 2 (Lmo2) is a transcription factor that plays a critical role in the development of T-acute lymphoblastic leukemia (T-ALL). A previous report established a link between Lmo2 expression and the nuclear presence of oncogenic Janus kinase 2 (JAK2), a non-receptor protein tyrosine kinase. The oncogenic JAK2 kinase phosphorylates histone H3 on Tyr 41 that leads to the relief of Lmo2 promoter repression and subsequent gene expression. Similar to JAK2, constitutive activation of lymphocyte-specific protein tyrosine kinase (Lck) has been implicated in lymphoid malignancies. However, it is not known whether oncogenic Lck regulates Lmo2 expression through a similar mechanism. We show here that Lmo2 expression is significantly elevated in T cell leukemia LSTRA overexpressing active Lck kinase and in HEK 293 cells expressing oncogenic Y505FLck kinase. Nuclear localization of active Lck kinase was confirmed in both Lck-transformed cells by subcellular fractionation and immunofluorescence microscopy. More importantly, in contrast to oncogenic JAK2, oncogenic Lck kinase does not result in significant increase in histone H3 phosphorylation on Tyr 41. Instead, chromatin immunoprecipitation experiment shows that oncogenic Y505FLck kinase binds to the Lmo2 promoter in vivo. This result raises the possibility that oncogenic Lck may activate Lmo2 promoter through direct interaction.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
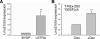
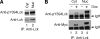
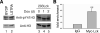
Similar articles
-
JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin.Nature. 2009 Oct 8;461(7265):819-22. doi: 10.1038/nature08448. Epub 2009 Sep 27. Nature. 2009. PMID: 19783980 Free PMC article.
-
Requirement for Lyl1 in a model of Lmo2-driven early T-cell precursor ALL.Blood. 2013 Sep 19;122(12):2093-103. doi: 10.1182/blood-2012-09-458570. Epub 2013 Aug 7. Blood. 2013. PMID: 23926305
-
LIM domain only-2 (LMO2) induces T-cell leukemia by two distinct pathways.PLoS One. 2014 Jan 21;9(1):e85883. doi: 10.1371/journal.pone.0085883. eCollection 2014. PLoS One. 2014. PMID: 24465765 Free PMC article.
-
LMO2 at 25 years: a paradigm of chromosomal translocation proteins.Open Biol. 2015 Jun;5(6):150062. doi: 10.1098/rsob.150062. Open Biol. 2015. PMID: 26108219 Free PMC article. Review.
-
Dysregulation of the protein tyrosine kinase LCK in lymphoproliferative disorders and in other neoplasias.Leuk Lymphoma. 1999 Oct;35(3-4):245-54. doi: 10.3109/10428199909145727. Leuk Lymphoma. 1999. PMID: 10706447 Review.
Cited by
-
Intracellular Signaling Pathways Involved in Childhood Acute Lymphoblastic Leukemia; Molecular _targets.Indian J Hematol Blood Transfus. 2016 Jun;32(2):141-53. doi: 10.1007/s12288-015-0609-z. Epub 2015 Oct 20. Indian J Hematol Blood Transfus. 2016. PMID: 27065575 Free PMC article. Review.
-
Down-regulation of the epithelial Na⁺ channel ENaC by Janus kinase 2.J Membr Biol. 2014 Apr;247(4):331-8. doi: 10.1007/s00232-014-9636-1. Epub 2014 Feb 23. J Membr Biol. 2014. PMID: 24562791
-
LCK facilitates DNA damage repair by stabilizing RAD51 and BRCA1 in the nucleus of chemoresistant ovarian cancer.J Ovarian Res. 2023 Jun 27;16(1):122. doi: 10.1186/s13048-023-01194-2. J Ovarian Res. 2023. PMID: 37370140 Free PMC article.
-
Nuclear lymphocyte-specific protein tyrosine kinase and its interaction with CR6-interacting factor 1 promote the survival of human leukemic T cells.Oncol Rep. 2015 Jul;34(1):43-50. doi: 10.3892/or.2015.3990. Epub 2015 May 19. Oncol Rep. 2015. PMID: 25997448 Free PMC article.
-
Up-regulation of Kv1.3 channels by janus kinase 2.J Membr Biol. 2015 Apr;248(2):309-17. doi: 10.1007/s00232-015-9772-2. Epub 2015 Feb 3. J Membr Biol. 2015. PMID: 25644777
References
-
- McCormack MP, Rabbitts TH. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2004;350:913–922. - PubMed
-
- Nam CH, Rabbitts TH. The role of LMO2 in development and in T cell leukemia after chromosomal translocation or retroviral insertion. Mol. Ther. 2006;13:15–25. - PubMed
-
- Oram SH, Thoms JA, Pridans C, Janes ME, Kinston SJ, Anand S, Landry JR, Lock RB, Jayaraman PS, Huntly BJ, Pimanda JE, Gottgens B. A previously unrecognized promoter of LMO2 forms part of a transcriptional regulatory circuit mediating LMO2 expression in a subset of T-acute lymphoblastic leukaemia patients. Oncogene. 2010;29:5796–5808. - PubMed
-
- Rabbitts TH, Axelson H, Forster A, Grutz G, Lavenir I, Larson R, Osada H, Valge-Archer V, Wadman I, Warren A. Chromosomal translocations and leukaemia: a role for LMO2 in T cell acute leukaemia, in transcription and in erythropoiesis. Leukemia. 1997;11(Suppl 3):271–272. - PubMed
-
- Landry JR, Kinston S, Knezevic K, Donaldson IJ, Green AR, Gottgens B. Fli1, Elf1, and Ets1 regulate the proximal promoter of the LMO2 gene in endothelial cells. Blood. 2005;106:2680–2687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

