The extracellular matrix: a dynamic niche in cancer progression
- PMID: 22351925
- PMCID: PMC3283993
- DOI: 10.1083/jcb.201102147
The extracellular matrix: a dynamic niche in cancer progression
Abstract
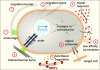
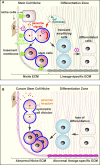
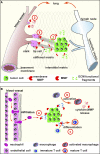
The local microenvironment, or niche, of a cancer cell plays important roles in cancer development. A major component of the niche is the extracellular matrix (ECM), a complex network of macromolecules with distinctive physical, biochemical, and biomechanical properties. Although tightly controlled during embryonic development and organ homeostasis, the ECM is commonly deregulated and becomes disorganized in diseases such as cancer. Abnormal ECM affects cancer progression by directly promoting cellular transformation and metastasis. Importantly, however, ECM anomalies also deregulate behavior of stromal cells, facilitate tumor-associated angiogenesis and inflammation, and thus lead to generation of a tumorigenic microenvironment. Understanding how ECM composition and topography are maintained and how their deregulation influences cancer progression may help develop new therapeutic interventions by _targeting the tumor niche.
Figures

Similar articles
-
The molecular composition of the metastatic niche.Exp Cell Res. 2013 Jul 1;319(11):1679-86. doi: 10.1016/j.yexcr.2013.04.017. Epub 2013 May 21. Exp Cell Res. 2013. PMID: 23707205 Review.
-
Extracellular matrix components in breast cancer progression and metastasis.Breast. 2013 Aug;22 Suppl 2:S66-72. doi: 10.1016/j.breast.2013.07.012. Breast. 2013. PMID: 24074795 Review.
-
Extracellular Matrix Alterations in Metastatic Processes.Int J Mol Sci. 2019 Oct 7;20(19):4947. doi: 10.3390/ijms20194947. Int J Mol Sci. 2019. PMID: 31591367 Free PMC article. Review.
-
Proinvasive extracellular matrix remodeling for tumor progression.Arch Pharm Res. 2019 Jan;42(1):40-47. doi: 10.1007/s12272-018-1097-0. Epub 2018 Dec 4. Arch Pharm Res. 2019. PMID: 30515725 Review.
-
Recapitulating the human tumor microenvironment: Colon tumor-derived extracellular matrix promotes angiogenesis and tumor cell growth.Biomaterials. 2017 Feb;116:118-129. doi: 10.1016/j.biomaterials.2016.11.034. Epub 2016 Nov 24. Biomaterials. 2017. PMID: 27914984 Free PMC article.
Cited by
-
Wentilactone A Reverses the NF-κB/ECM1 Signaling-Induced Cisplatin Resistance through Inhibition of IKK/IκB in Ovarian Cancer Cells.Nutrients. 2022 Sep 14;14(18):3790. doi: 10.3390/nu14183790. Nutrients. 2022. PMID: 36145166 Free PMC article.
-
Combination of Quantitative Parameters of Shear Wave Elastography and Superb Microvascular Imaging to Evaluate Breast Masses.Korean J Radiol. 2020 Sep;21(9):1045-1054. doi: 10.3348/kjr.2019.0765. Korean J Radiol. 2020. PMID: 32691540 Free PMC article.
-
The association between laminin and microglial morphology in vitro.Sci Rep. 2016 Jun 23;6:28580. doi: 10.1038/srep28580. Sci Rep. 2016. PMID: 27334934 Free PMC article.
-
The updated landscape of tumor microenvironment and drug repurposing.Signal Transduct _target Ther. 2020 Aug 25;5(1):166. doi: 10.1038/s41392-020-00280-x. Signal Transduct _target Ther. 2020. PMID: 32843638 Free PMC article. Review.
-
Neural regulation of cancer: from mechanobiology to inflammation.Clin Transl Immunology. 2016 May 13;5(5):e78. doi: 10.1038/cti.2016.18. eCollection 2016 May. Clin Transl Immunology. 2016. PMID: 27350878 Free PMC article. Review.
References
-
- Akhtar N., Marlow R., Lambert E., Schatzmann F., Lowe E.T., Cheung J., Katz E., Li W., Wu C., Dedhar S., et al. 2009. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development. 136:1019–1027 10.1242/dev.028423 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

