Hematopoietic stem cell transplantation improves the high incidence of neutralizing allo-antibodies observed in Hurler's syndrome after pharmacological enzyme replacement therapy
- PMID: 22371174
- PMCID: PMC3436232
- DOI: 10.3324/haematol.2011.058644
Hematopoietic stem cell transplantation improves the high incidence of neutralizing allo-antibodies observed in Hurler's syndrome after pharmacological enzyme replacement therapy
Abstract
Background: Mucopolysaccharidosis type I is caused by deficiency of α-L-iduronidase. Currently available treatment options include an allogeneic hematopoietic stem cell transplant and enzyme replacement therapy. Exogenous enzyme therapy appears promising but the benefits may be attenuated, at least in some patients, by the development of an immune response to the delivered enzyme. The incidence and impact of alloimmune responses in these patients remain unknown.
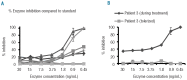
Design and methods: We developed an immunoglobulin G enzyme-linked immunosorbent assay as well as in vitro catalytic enzyme inhibition and cellular uptake inhibition assays and quantified enzyme inhibition by allo-antibodies. We determined the impact of these antibodies in eight patients who received enzyme therapy before and during hematopoietic stem cell transplantation. In addition, 20 patients who had previously received an allogeneic stem cell transplant were tested to evaluate this treatment as an immune tolerance induction mechanism.
Results: High titer immune responses were seen in 87.5% (7/8) patients following exposure to α-L-iduronidase. These patients exhibited catalytic enzyme inhibition (5/8), uptake inhibition of catalytically active enzyme (6/8) or both (4/8). High antibody titers generally preceded elevation of previously described biomarkers of disease progression. The median time to development of immune tolerance was 101 days (range, 26-137) after transplantation. All 20 patients, including those with mixed chimerism (22%), tested 1 year after transplantation were tolerized despite normal enzyme levels.
Conclusions: We found a high incidence of neutralizing antibodies in patients with mucopolysaccharidosis type I treated with enzyme replacement therapy. We also found that allogeneic hematopoietic stem cell transplantation was an effective and rapid immune tolerance induction strategy.
Figures



Similar articles
-
Cord-blood transplants from unrelated donors in patients with Hurler's syndrome.N Engl J Med. 2004 May 6;350(19):1960-9. doi: 10.1056/NEJMoa032613. N Engl J Med. 2004. PMID: 15128896
-
Biomarker responses correlate with antibody status in mucopolysaccharidosis type I patients on long-term enzyme replacement therapy.Mol Genet Metab. 2015 Feb;114(2):129-37. doi: 10.1016/j.ymgme.2014.10.012. Epub 2014 Oct 29. Mol Genet Metab. 2015. PMID: 25467058
-
Enzyme replacement therapy prior to haematopoietic stem cell transplantation in Mucopolysaccharidosis Type I: 10 year combined experience of 2 centres.Mol Genet Metab. 2016 Mar;117(3):373-7. doi: 10.1016/j.ymgme.2016.01.011. Epub 2016 Jan 27. Mol Genet Metab. 2016. PMID: 26832957 Free PMC article.
-
Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I.Lancet. 2003 May 10;361(9369):1608-13. doi: 10.1016/S0140-6736(03)13311-9. Lancet. 2003. PMID: 12747881 Review.
-
Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure.Orphanet J Rare Dis. 2011 Aug 10;6:55. doi: 10.1186/1750-1172-6-55. Orphanet J Rare Dis. 2011. PMID: 21831279 Free PMC article.
Cited by
-
Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome.Orphanet J Rare Dis. 2015 Apr 10;10:42. doi: 10.1186/s13023-015-0255-4. Orphanet J Rare Dis. 2015. PMID: 25887468 Free PMC article.
-
Plasma and urinary levels of dermatan sulfate and heparan sulfate derived disaccharides after long-term enzyme replacement therapy (ERT) in MPS I: correlation with the timing of ERT and with total urinary excretion of glycosaminoglycans.J Inherit Metab Dis. 2013 Mar;36(2):247-55. doi: 10.1007/s10545-012-9538-2. Epub 2012 Sep 19. J Inherit Metab Dis. 2013. PMID: 22991166
-
Post-transplant laronidase augmentation for children with Hurler syndrome: biochemical outcomes.Sci Rep. 2019 Oct 1;9(1):14105. doi: 10.1038/s41598-019-50595-1. Sci Rep. 2019. PMID: 31575939 Free PMC article. Clinical Trial.
-
Gene Therapy Developments for Pompe Disease.Biomedicines. 2022 Jan 28;10(2):302. doi: 10.3390/biomedicines10020302. Biomedicines. 2022. PMID: 35203513 Free PMC article. Review.
-
Immune responses to cells and proteins after hematopoietic stem cell gene therapy for inherited diseases: A cause for concern.Mol Ther. 2024 Oct 2;32(10):3201-3202. doi: 10.1016/j.ymthe.2024.09.014. Epub 2024 Sep 17. Mol Ther. 2024. PMID: 39293430 No abstract available.
References
-
- Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, et al. Replacement therapy for inherited enzyme deficiency–macrophage-_targeted gluco-cerebrosidase for Gaucher’s disease. N Engl J Med. 1991;324(21):1464–70. - PubMed
-
- Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al. A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1. Health Technol Assess. 2006;10(20):iii–iv. ix, 113. - PubMed
-
- Porter S. Human immune response to recombinant human proteins. J Pharm Sci. 2001;90(1):1–11. - PubMed
-
- Clarke LA, Wraith JE, Beck M, Kolodny EH, Pastores GM, Muenzer J, et al. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123(1):229–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

