Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca²+- and K+-permeable conductance in root cells
- PMID: 22523205
- PMCID: PMC3398561
- DOI: 10.1105/tpc.112.097881
Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca²+- and K+-permeable conductance in root cells
Abstract
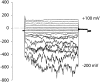
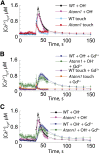
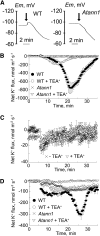
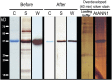
Plant cell growth and stress signaling require Ca²⁺ influx through plasma membrane transport proteins that are regulated by reactive oxygen species. In root cell growth, adaptation to salinity stress, and stomatal closure, such proteins operate downstream of the plasma membrane NADPH oxidases that produce extracellular superoxide anion, a reactive oxygen species that is readily converted to extracellular hydrogen peroxide and hydroxyl radicals, OH•. In root cells, extracellular OH• activates a plasma membrane Ca²⁺-permeable conductance that permits Ca²⁺ influx. In Arabidopsis thaliana, distribution of this conductance resembles that of annexin1 (ANN1). Annexins are membrane binding proteins that can form Ca²⁺-permeable conductances in vitro. Here, the Arabidopsis loss-of-function mutant for annexin1 (Atann1) was found to lack the root hair and epidermal OH•-activated Ca²⁺- and K⁺-permeable conductance. This manifests in both impaired root cell growth and ability to elevate root cell cytosolic free Ca²⁺ in response to OH•. An OH•-activated Ca²⁺ conductance is reconstituted by recombinant ANN1 in planar lipid bilayers. ANN1 therefore presents as a novel Ca²⁺-permeable transporter providing a molecular link between reactive oxygen species and cytosolic Ca²⁺ in plants.
Figures



Similar articles
-
Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells.J Cell Sci. 2003 Jan 1;116(Pt 1):81-8. doi: 10.1242/jcs.00201. J Cell Sci. 2003. PMID: 12456718
-
Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots.Plant J. 2014 Jan;77(1):136-45. doi: 10.1111/tpj.12372. Epub 2013 Nov 29. Plant J. 2014. PMID: 24180429
-
Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth.Plant J. 2002 Dec;32(5):799-808. doi: 10.1046/j.1365-313x.2002.01467.x. Plant J. 2002. PMID: 12472694
-
Genes for calcium-permeable channels in the plasma membrane of plant root cells.Biochim Biophys Acta. 2002 Aug 31;1564(2):299-309. doi: 10.1016/s0005-2736(02)00509-6. Biochim Biophys Acta. 2002. PMID: 12175911 Review.
-
Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: implications for plant adaptive responses.J Exp Bot. 2014 Mar;65(5):1271-83. doi: 10.1093/jxb/ert423. Epub 2014 Jan 24. J Exp Bot. 2014. PMID: 24465010 Review.
Cited by
-
Plant signaling networks involving Ca(2+) and Rboh/Nox-mediated ROS production under salinity stress.Front Plant Sci. 2015 Jun 10;6:427. doi: 10.3389/fpls.2015.00427. eCollection 2015. Front Plant Sci. 2015. PMID: 26113854 Free PMC article. Review.
-
Calcium-Mediated Abiotic Stress Signaling in Roots.Front Plant Sci. 2016 Aug 29;7:1296. doi: 10.3389/fpls.2016.01296. eCollection 2016. Front Plant Sci. 2016. PMID: 27621742 Free PMC article. Review.
-
Genome-wide analysis of annexin gene family in Schrenkiella parvula and Eutrema salsugineum suggests their roles in salt stress response.PLoS One. 2023 Jan 18;18(1):e0280246. doi: 10.1371/journal.pone.0280246. eCollection 2023. PLoS One. 2023. PMID: 36652493 Free PMC article.
-
Plant salt-tolerance mechanisms.Trends Plant Sci. 2014 Jun;19(6):371-9. doi: 10.1016/j.tplants.2014.02.001. Epub 2014 Mar 14. Trends Plant Sci. 2014. PMID: 24630845 Free PMC article. Review.
-
Calcium and reactive oxygen species rule the waves of signaling.Plant Physiol. 2013 Oct;163(2):471-85. doi: 10.1104/pp.113.222950. Epub 2013 Jul 29. Plant Physiol. 2013. PMID: 23898042 Free PMC article. Review.
References
-
- Alexandersson E., Saalbach G., Larsson C., Kjellbom P. (2004). Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol. 45: 1543–1556 - PubMed
-
- Balasubramanian K., Bevers E.M., Willems G.M., Schroit A.J. (2001). Binding of annexin V to membrane products of lipid peroxidation. Biochemistry 40: 8672–8676 - PubMed
-
- Benschop J.J., Mohammed S., O’Flaherty M., Heck A.J.R., Slijper M., Menke F.L.H. (2007). Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol. Cell. Proteomics 6: 1198–1214 - PubMed
-
- Burger A., Voges D., Demange P., Perez C.R., Huber R., Berendes R. (1994). Structural and electrophysiological analysis of annexin V mutants. Mutagenesis of human annexin V, an in vitro voltage-gated calcium channel, provides information about the structural features of the ion pathway, the voltage sensor and the ion selectivity filter. J. Mol. Biol. 237: 479–499 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

