Stress-induced sensitization of cortical adrenergic receptors following a history of cannabinoid exposure
- PMID: 22677142
- PMCID: PMC3905974
- DOI: 10.1016/j.expneurol.2012.05.016
Stress-induced sensitization of cortical adrenergic receptors following a history of cannabinoid exposure
Abstract
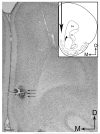
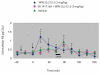
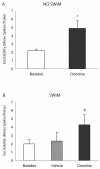
The cannabinoid receptor agonist, WIN 55,212-2, increases extracellular norepinephrine levels in the rat frontal cortex under basal conditions, likely via desensitization of inhibitory α2-adrenergic receptors located on norepinephrine terminals. Here, the effect of WIN 55,212-2 on stress-induced norepinephrine release was assessed in the medial prefrontal cortex (mPFC), in adult male Sprague-Dawley rats using in vivo microdialysis. Systemic administration of WIN 55,212-2 30 min prior to stressor exposure prevented stress-induced cortical norepinephrine release induced by a single exposure to swim when compared to vehicle. To further probe cortical cannabinoid-adrenergic interactions, postsynaptic α2-adrenergic receptor (AR)-mediated responses were assessed in mPFC pyramidal neurons using electrophysiological analysis in an in vitro cortical slice preparation. We confirm prior studies showing that clonidine increases cortical pyramidal cell excitability and that this was unaffected by exposure to acute stress. WIN 55,212-2, via bath application, blocked postsynaptic α2-AR mediated responses in cortical neurons irrespective of exposure to stress. Interestingly, stress exposure prevented the desensitization of α2-AR mediated responses produced by a history of cannabinoid exposure. Together, these data indicate the stress-dependent nature of cannabinoid interactions via both pre- and postsynaptic ARs. In summary, microdialysis data indicate that cannabinoids restrain stress-induced cortical NE efflux. Electrophysiology data indicate that cannabinoids also restrain cortical cell excitability under basal conditions; however, stress interferes with these CB1-α2 AR interactions, potentially contributing to over-activation of pyramidal neurons in mPFC. Overall, cannabinoids are protective of the NE system and cortical excitability but stress can derail this protective effect, potentially contributing to stress-related psychopathology. These data add to the growing evidence of complex, stress-dependent modulation of monoaminergic systems by cannabinoids and support the potential use of cannabinoids in the treatment of stress-induced noradrenergic dysfunction.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
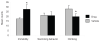

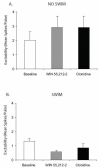
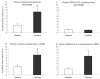
Comment in
-
Modulating the modulators: interaction of brain norepinephrine and cannabinoids in stress.Exp Neurol. 2012 Dec;238(2):145-8. doi: 10.1016/j.expneurol.2012.08.016. Epub 2012 Aug 19. Exp Neurol. 2012. PMID: 22981451 Free PMC article. No abstract available.
Similar articles
-
Cortical adrenoceptor expression, function and adaptation under conditions of cannabinoid receptor deletion.Exp Neurol. 2017 Jun;292:179-192. doi: 10.1016/j.expneurol.2017.03.010. Epub 2017 Mar 21. Exp Neurol. 2017. PMID: 28341460 Free PMC article.
-
Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex.Eur J Neurosci. 2014 Oct;40(8):3202-14. doi: 10.1111/ejn.12690. Epub 2014 Aug 18. Eur J Neurosci. 2014. PMID: 25131562 Free PMC article.
-
Cannabinoid modulation of cortical adrenergic receptors and transporters.J Neurosci Res. 2009 Dec;87(16):3671-8. doi: 10.1002/jnr.22158. J Neurosci Res. 2009. PMID: 19533736 Free PMC article.
-
Modulating the modulators: interaction of brain norepinephrine and cannabinoids in stress.Exp Neurol. 2012 Dec;238(2):145-8. doi: 10.1016/j.expneurol.2012.08.016. Epub 2012 Aug 19. Exp Neurol. 2012. PMID: 22981451 Free PMC article. No abstract available.
-
The stoned age: Sex differences in the effects of adolescent cannabinoid exposure on prefrontal cortex structure and function in animal models.Int Rev Neurobiol. 2022;161:121-145. doi: 10.1016/bs.irn.2021.07.005. Epub 2021 Aug 3. Int Rev Neurobiol. 2022. PMID: 34801167 Free PMC article. Review.
Cited by
-
Jieyu Anshen Granule, a Chinese Herbal Formulation, Exerts Effects on Poststroke Depression in Rats.Evid Based Complement Alternat Med. 2020 Feb 23;2020:7469068. doi: 10.1155/2020/7469068. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32184899 Free PMC article.
-
Cortical adrenoceptor expression, function and adaptation under conditions of cannabinoid receptor deletion.Exp Neurol. 2017 Jun;292:179-192. doi: 10.1016/j.expneurol.2017.03.010. Epub 2017 Mar 21. Exp Neurol. 2017. PMID: 28341460 Free PMC article.
-
Interaction of Cannabis Use and Aging: From Molecule to Mind.J Dual Diagn. 2020 Jan-Mar;16(1):140-176. doi: 10.1080/15504263.2019.1665218. Epub 2019 Sep 30. J Dual Diagn. 2020. PMID: 31570066 Free PMC article. Review.
-
Co-localization of the cannabinoid type 1 receptor with corticotropin-releasing factor-containing afferents in the noradrenergic nucleus locus coeruleus: implications for the cognitive limb of the stress response.Brain Struct Funct. 2017 Sep;222(7):3007-3023. doi: 10.1007/s00429-017-1381-7. Epub 2017 Mar 2. Brain Struct Funct. 2017. PMID: 28255675 Free PMC article.
-
Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex.Eur J Neurosci. 2014 Oct;40(8):3202-14. doi: 10.1111/ejn.12690. Epub 2014 Aug 18. Eur J Neurosci. 2014. PMID: 25131562 Free PMC article.
References
-
- Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur. J. Pharmacol. 2000;401:179–185. - PubMed
-
- Alger BE, Dhanjal SS, Dingledine R, Garthwaite J, Henderson G, King GL. In: Brain slice methods. Dingledine R, editor. Brain slice Plenum Press; New York, New York: 1984. pp. 381–438.
-
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

