Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice
- PMID: 22768070
- PMCID: PMC3387145
- DOI: 10.1371/journal.pone.0039286
Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice
Abstract
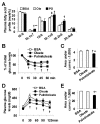
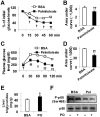
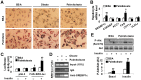
The interaction between fat deposition and inflammation during obesity contributes to the development of non-alcoholic fatty liver disease (NAFLD). The present study examined the effects of palmitoleate, a monounsaturated fatty acid (16:1n7), on liver metabolic and inflammatory responses, and investigated the mechanisms by which palmitoleate increases hepatocyte fatty acid synthase (FAS) expression. Male wild-type C57BL/6J mice were supplemented with palmitoleate and subjected to the assays to analyze hepatic steatosis and liver inflammatory response. Additionally, mouse primary hepatocytes were treated with palmitoleate and used to analyze fat deposition, the inflammatory response, and sterol regulatory element-binding protein 1c (SREBP1c) activation. Compared with controls, palmitoleate supplementation increased the circulating levels of palmitoleate and improved systemic insulin sensitivity. Locally, hepatic fat deposition and SREBP1c and FAS expression were significantly increased in palmitoleate-supplemented mice. These pro-lipogenic events were accompanied by improvement of liver insulin signaling. In addition, palmitoleate supplementation reduced the numbers of macrophages/Kupffer cells in livers of the treated mice. Consistently, supplementation of palmitoleate decreased the phosphorylation of nuclear factor kappa B (NF-κB, p65) and the expression of proinflammatory cytokines. These results were recapitulated in primary mouse hepatocytes. In terms of regulating FAS expression, treatment of palmitoleate increased the transcription activity of SREBP1c and enhanced the binding of SREBP1c to FAS promoter. Palmitoleate also decreased the phosphorylation of NF-κB p65 and the expression of proinflammatory cytokines in cultured macrophages. Together, these results suggest that palmitoleate acts through dissociating liver inflammatory response from hepatic steatosis to play a unique role in NAFLD.
Conflict of interest statement
Figures


Similar articles
-
Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity.PLoS One. 2014 Mar 17;9(3):e91111. doi: 10.1371/journal.pone.0091111. eCollection 2014. PLoS One. 2014. PMID: 24638078 Free PMC article.
-
Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity.Hepatology. 2018 Jul;68(1):48-61. doi: 10.1002/hep.29777. Epub 2018 May 10. Hepatology. 2018. PMID: 29315766 Free PMC article.
-
FoxO3 regulates hepatic triglyceride metabolism via modulation of the expression of sterol regulatory-element binding protein 1c.Lipids Health Dis. 2019 Nov 15;18(1):197. doi: 10.1186/s12944-019-1132-2. Lipids Health Dis. 2019. PMID: 31729980 Free PMC article.
-
The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease.Adv Nutr. 2017 Jan 17;8(1):173S-181S. doi: 10.3945/an.115.011130. Print 2017 Jan. Adv Nutr. 2017. PMID: 28096141 Free PMC article. Review.
-
Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise.Cell Mol Life Sci. 2006 Jun;63(12):1393-409. doi: 10.1007/s00018-006-6600-y. Cell Mol Life Sci. 2006. PMID: 16649140 Free PMC article. Review.
Cited by
-
PFKFB3 Control of Cancer Growth by Responding to Circadian Clock Outputs.Sci Rep. 2016 Apr 15;6:24324. doi: 10.1038/srep24324. Sci Rep. 2016. PMID: 27079271 Free PMC article.
-
The Novel Perspectives of Adipokines on Brain Health.Int J Mol Sci. 2019 Nov 11;20(22):5638. doi: 10.3390/ijms20225638. Int J Mol Sci. 2019. PMID: 31718027 Free PMC article. Review.
-
Mice lacking adenosine 2A receptor reveal increased severity of MCD-induced NASH.J Endocrinol. 2019 Sep 1:JOE-19-0198.R1. doi: 10.1530/JOE-19-0198. Online ahead of print. J Endocrinol. 2019. PMID: 31505462 Free PMC article.
-
Isoquercetin Improves Hepatic Lipid Accumulation by Activating AMPK Pathway and Suppressing TGF-β Signaling on an HFD-Induced Nonalcoholic Fatty Liver Disease Rat Model.Int J Mol Sci. 2018 Dec 19;19(12):4126. doi: 10.3390/ijms19124126. Int J Mol Sci. 2018. PMID: 30572631 Free PMC article.
-
Dietary fat sources differentially modulate intestinal barrier and hepatic inflammation in alcohol-induced liver injury in rats.Am J Physiol Gastrointest Liver Physiol. 2013 Dec;305(12):G919-32. doi: 10.1152/ajpgi.00226.2013. Epub 2013 Oct 10. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 24113767 Free PMC article.
References
-
- Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2, 46–53. 2005. - PubMed
-
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 52, 1836–1846. 2010. - PubMed
-
- Tilg H, Kaser A. Treatment strategies in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2, 148–155. 2005. - PubMed
-
- Marchesini G, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet 358, 893–894. 2001. - PubMed
-
- Angulo P. NAFLD, Obesity, and Bariatric Surgery. Gastroenterology 130, 1848–1852. 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

