Heightened adaptive immune responses following vaccination with a temperature-sensitive, live-attenuated influenza virus compared to adjuvanted, whole-inactivated virus in pigs
- PMID: 22835742
- PMCID: PMC3743435
- DOI: 10.1016/j.vaccine.2012.07.033
Heightened adaptive immune responses following vaccination with a temperature-sensitive, live-attenuated influenza virus compared to adjuvanted, whole-inactivated virus in pigs
Abstract
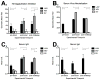
In the United States there are currently two influenza vaccine platforms approved for use in humans-conventional inactivated virus and live-attenuated influenza virus (LAIV). One of the major challenges for influenza A virus (IAV) vaccination is designing a platform that provides protection across strains. Pandemic H1N1 (pH1N1) IAV swept the globe in 2009 and crossed the species barrier, infecting swine in several countries. Pigs are a natural host for IAV and serve as a model for evaluating immune responses following vaccination and challenge. Recently, a temperature-sensitive (ts) LAIV was developed by introducing modifications in the polymerase genes of a swine-like triple reassortant (tr) virus and when paired with pandemic HA and NA, provided sterilizing immunity upon intratracheal challenge with virulent pH1N1 virus. The utility of a ts LAIV is expanded in this report to show vaccination of pigs induced a cell-mediated immune response characterized by an increased number of antigen-specific IFN-secreting cells and expanded T cell populations when compared to pigs vaccinated with a whole inactivated virus (WIV) vaccine. Following challenge, there was a significant increase in the percentage of proliferating lymphocytes in the LAIV group compared to the WIV group following restimulation with pH1N1 in vitro. Also, there was an increase in the percentage of CD4/CD8 double-positive memory T cells in LAIV vaccinated pigs compared to WIV vaccinated pigs. Hemagglutination inhibition and serum neutralization titers were significantly higher in the LAIV-vaccinated pigs compared to the WIV vaccinated pigs following the initial dose of vaccine. Taken together, these results indicate the ts LAIV vaccine, generated from a triple reassortant IAV, elicits greater cell-mediated and humoral immune responses in pigs.
Published by Elsevier Ltd.
Figures


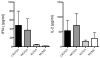
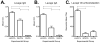
Similar articles
-
Factors affecting induction of peripheral IFN-γ recall response to influenza A virus vaccination in pigs.Vet Immunol Immunopathol. 2017 Mar;185:57-65. doi: 10.1016/j.vetimm.2017.01.009. Epub 2017 Feb 5. Vet Immunol Immunopathol. 2017. PMID: 28242003
-
Age at Vaccination and Timing of Infection Do Not Alter Vaccine-Associated Enhanced Respiratory Disease in Influenza A Virus-Infected Pigs.Clin Vaccine Immunol. 2016 Jun 6;23(6):470-482. doi: 10.1128/CVI.00563-15. Print 2016 Jun. Clin Vaccine Immunol. 2016. PMID: 27030585 Free PMC article.
-
Vaccination of pigs with a codon-pair bias de-optimized live attenuated influenza vaccine protects from homologous challenge.Vaccine. 2018 Feb 14;36(8):1101-1107. doi: 10.1016/j.vaccine.2018.01.027. Vaccine. 2018. PMID: 29366707
-
Novel Approaches for The Development of Live Attenuated Influenza Vaccines.Viruses. 2019 Feb 22;11(2):190. doi: 10.3390/v11020190. Viruses. 2019. PMID: 30813325 Free PMC article. Review.
-
Immune responses after live attenuated influenza vaccination.Hum Vaccin Immunother. 2018 Mar 4;14(3):571-578. doi: 10.1080/21645515.2017.1377376. Epub 2018 Jan 3. Hum Vaccin Immunother. 2018. PMID: 28933664 Free PMC article. Review.
Cited by
-
Short- and long-term protective efficacy against clade 2.3.4.4 H5N2 highly pathogenic avian influenza virus following prime-boost vaccination in turkeys.Vaccine. 2017 Oct 9;35(42):5637-5643. doi: 10.1016/j.vaccine.2017.08.059. Epub 2017 Sep 6. Vaccine. 2017. PMID: 28886943 Free PMC article.
-
Current and prospective control strategies of influenza A virus in swine.Porcine Health Manag. 2021 Feb 28;7(1):23. doi: 10.1186/s40813-021-00196-0. Porcine Health Manag. 2021. PMID: 33648602 Free PMC article. Review.
-
Universal Vaccines and Vaccine Platforms to Protect against Influenza Viruses in Humans and Agriculture.Front Microbiol. 2018 Feb 6;9:123. doi: 10.3389/fmicb.2018.00123. eCollection 2018. Front Microbiol. 2018. PMID: 29467737 Free PMC article. Review.
-
Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies.Front Vet Sci. 2020 Sep 22;7:647. doi: 10.3389/fvets.2020.00647. eCollection 2020. Front Vet Sci. 2020. PMID: 33195504 Free PMC article. Review.
-
African Swine Fever Modified Live Vaccine Candidates: Transitioning from Discovery to Product Development through Harmonized Standards and Guidelines.Viruses. 2022 Nov 24;14(12):2619. doi: 10.3390/v14122619. Viruses. 2022. PMID: 36560623 Free PMC article. Review.
References
-
- Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses a North American perspective. Adv Virus Res. 2008;72:127–54. - PubMed
-
- Vincent AL, Ma W, Lager KM, Gramer MR, Richt JA, Janke BH. Characterization of a newly emerged genetic cluster of H1N1 and H1N2 swine influenza virus in the United States. Virus genes. 2009 Jul 14; - PubMed
-
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. The New England journal of medicine. 2009 Jun 18;360(25):2605–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

