3-phosphoinositide-dependent kinase 1 controls breast tumor growth in a kinase-dependent but Akt-independent manner
- PMID: 22952425
- PMCID: PMC3431179
- DOI: 10.1593/neo.12856
3-phosphoinositide-dependent kinase 1 controls breast tumor growth in a kinase-dependent but Akt-independent manner
Abstract
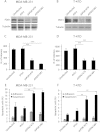
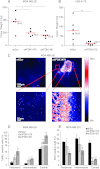
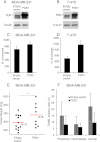
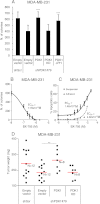
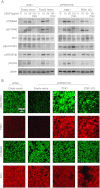
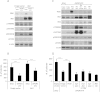
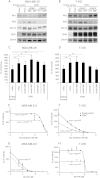
3-phosphoinositide-dependent protein kinase 1 (PDK1) is the pivotal element of the phosphatidylinositol 3 kinase (PI3K) signaling pathway because it phosphorylates Akt/PKB through interactions with phosphatidylinositol 3,4,5 phosphate. Recent data indicate that PDK1 is overexpressed in many breast carcinomas and that alterations of PDK1 are critical in the context of oncogenic PI3K activation. However, the role of PDK1 in tumor progression is still controversial. Here, we show that PDK1 is required for anchorage-independent and xenograft growth of breast cancer cells harboring either PI3KCA or KRAS mutations. In fact, PDK1 silencing leads to increased anoikis, reduced soft agar growth, and pronounced apoptosis inside tumors. Interestingly, these phenotypes are reverted by PDK1 wild-type but not kinase-dead mutant, suggesting a relevant role of PDK1 kinase activity, even if PDK1 is not relevant for Akt activation here. Indeed, the expression of constitutively active forms of Akt in PDK1 knockdown cells is unable to rescue the anchorage-independent growth. In addition, Akt down-regulation and pharmacological inhibition do not inhibit the effects of PDK1 overexpression. In summary, these results suggest that PDK1 may contribute to breast cancer, even in the absence of PI3K oncogenic mutations and through both Akt-dependent and Akt-independent mechanisms.
Figures
Similar articles
-
RET/PTC (rearranged in transformation/papillary thyroid carcinomas) tyrosine kinase phosphorylates and activates phosphoinositide-dependent kinase 1 (PDK1): an alternative phosphatidylinositol 3-kinase-independent pathway to activate PDK1.Mol Endocrinol. 2003 Jul;17(7):1382-94. doi: 10.1210/me.2002-0402. Epub 2003 May 8. Mol Endocrinol. 2003. PMID: 12738763
-
Runx2 activates PI3K/Akt signaling via mTORC2 regulation in invasive breast cancer cells.Breast Cancer Res. 2014 Jan 30;16(1):R16. doi: 10.1186/bcr3611. Breast Cancer Res. 2014. PMID: 24479521 Free PMC article.
-
IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation.J Biol Chem. 2011 Oct 28;286(43):37389-98. doi: 10.1074/jbc.M111.287433. Epub 2011 Sep 9. J Biol Chem. 2011. Retraction in: J Biol Chem. 2016 Oct 21;291(43):22853. doi: 10.1074/jbc.A111.287433 PMID: 21908616 Free PMC article. Retracted.
-
_targeting the phosphatidylinositol 3-kinase signaling pathway in breast cancer.Oncologist. 2011;16(4):404-14. doi: 10.1634/theoncologist.2010-0402. Epub 2011 Mar 15. Oncologist. 2011. PMID: 21406469 Free PMC article. Review.
-
Small-molecule inhibitors of PDK1.ChemMedChem. 2008 Dec;3(12):1810-38. doi: 10.1002/cmdc.200800195. ChemMedChem. 2008. PMID: 18972468 Review.
Cited by
-
PI3K signaling in cancer: beyond AKT.Curr Opin Cell Biol. 2017 Apr;45:62-71. doi: 10.1016/j.ceb.2017.02.007. Epub 2017 Mar 24. Curr Opin Cell Biol. 2017. PMID: 28343126 Free PMC article. Review.
-
AKT-independent PI3-K signaling in cancer - emerging role for SGK3.Cancer Manag Res. 2013 Aug 26;5:281-92. doi: 10.2147/CMAR.S35178. eCollection 2013. Cancer Manag Res. 2013. PMID: 24009430 Free PMC article. Review.
-
PI3K and AKT: Unfaithful Partners in Cancer.Int J Mol Sci. 2015 Sep 3;16(9):21138-52. doi: 10.3390/ijms160921138. Int J Mol Sci. 2015. PMID: 26404259 Free PMC article. Review.
-
The tumor suppressor PTEN and the PDK1 kinase regulate formation of the columnar neural epithelium.Elife. 2016 Jan 26;5:e12034. doi: 10.7554/eLife.12034. Elife. 2016. PMID: 26809587 Free PMC article.
-
Overcoming intratumor heterogeneity of polygenic cancer drug resistance with improved biomarker integration.Neoplasia. 2012 Dec;14(12):1278-89. doi: 10.1593/neo.122096. Neoplasia. 2012. PMID: 23308059 Free PMC article.
References
-
- Baselga J. _targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(suppl 1):12–19. - PubMed
-
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. - PubMed
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
