Leukotriene D4 and interleukin-13 cooperate to increase the release of eotaxin-3 by airway epithelial cells
- PMID: 22952702
- PMCID: PMC3432028
- DOI: 10.1371/journal.pone.0043544
Leukotriene D4 and interleukin-13 cooperate to increase the release of eotaxin-3 by airway epithelial cells
Abstract
Introduction: Airway epithelial cells play a central role in the physiopathology of asthma. They release eotaxins when treated with T(H)2 cytokines such as interleukin (IL)-4 or IL-13, and these chemokines attract eosinophils and potentiate the biosynthesis of cysteinyl leukotrienes (cysLTs), which in turn induce bronchoconstriction and mucus secretion. These effects of cysLTs mainly mediated by CysLT(1) and CysLT(2) receptors on epithelial cell functions remain largely undefined. Because the release of inflammatory cytokines, eotaxins, and cysLTs occur relatively at the same time and location in the lung tissue, we hypothesized that they regulate inflammation cooperatively rather than redundantly. We therefore investigated whether cysLTs and the T(H)2 cytokines would act in concert to augment the release of eotaxins by airway epithelial cells.
Methods: A549 cells or human primary bronchial epithelial cells were incubated with or without IL-4, IL-13, and/or LTD(4). The release of eotaxin-3 and the expression of cysLT receptors were assessed by ELISA, RT-PCR, and flow cytometry, respectively.
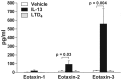
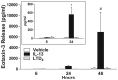
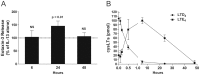
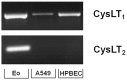
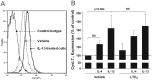
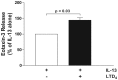
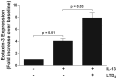
Results: IL-4 and IL-13 induced the release of eotaxin-3 by airway epithelial cells. LTD(4) weakly induced the release of eotaxin-3 but clearly potentiated the IL-13-induced eotaxin-3 release. LTD(4) had no effect on IL-4-stimulated cells. Epithelial cells expressed CysLT(1) but not CysLT(2). CysLT(1) expression was increased by IL-13 but not by IL-4 and/or LTD(4). Importantly, the upregulation of CysLT(1) by IL-13 preceded eotaxin-3 release.
Conclusions: These results demonstrate a stepwise cooperation between IL-13 and LTD(4). IL-13 upregulates CysLT(1) expression and consequently the response to cysLTs This results in an increased release of eotaxin-3 by epithelial cells which at its turn increases the recruitment of leukocytes and their biosynthesis of cysLTs. This positive amplification loop involving epithelial cells and leukocytes could be implicated in the recruitment of eosinophils observed in asthmatics.
Conflict of interest statement
Figures
Similar articles
-
Up-regulation of cysteinyl leukotriene 1 receptor by IL-13 enables human lung fibroblasts to respond to leukotriene C4 and produce eotaxin.J Immunol. 2003 Apr 15;170(8):4290-5. doi: 10.4049/jimmunol.170.8.4290. J Immunol. 2003. PMID: 12682264
-
Cytokine-stimulated human lung alveolar epithelial cells release eotaxin-2 (CCL24) and eotaxin-3 (CCL26).J Interferon Cytokine Res. 2005 Feb;25(2):82-91. doi: 10.1089/jir.2005.25.82. J Interferon Cytokine Res. 2005. PMID: 15695929
-
Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1.J Allergy Clin Immunol. 2012 Apr;129(4):1136-42. doi: 10.1016/j.jaci.2012.01.057. Epub 2012 Mar 3. J Allergy Clin Immunol. 2012. PMID: 22391114
-
The roles of cysteinyl leukotrienes in eosinophilic inflammation of asthmatic airways.Int Arch Allergy Immunol. 2003 Jun;131 Suppl 1:7-10. doi: 10.1159/000070474. Int Arch Allergy Immunol. 2003. PMID: 12771542 Review.
-
Cysteinyl leukotrienes and their receptors: molecular and functional characteristics.Pharmacology. 2010;85(6):336-49. doi: 10.1159/000312669. Epub 2010 Jun 2. Pharmacology. 2010. PMID: 20516735 Review.
Cited by
-
Petiveria alliacea Suppresses Airway Inflammation and Allergen-Specific Th2 Responses in Ovalbumin-Sensitized Murine Model of Asthma.Chin J Integr Med. 2018 Dec;24(12):912-919. doi: 10.1007/s11655-018-2566-5. Epub 2018 Oct 19. Chin J Integr Med. 2018. PMID: 30341485
-
Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7.Br J Pharmacol. 2014 Aug;171(15):3551-74. doi: 10.1111/bph.12665. Epub 2014 Jul 12. Br J Pharmacol. 2014. PMID: 24588652 Free PMC article. Review.
-
Ethanol Extract of Perilla frutescens Suppresses Allergen-Specific Th2 Responses and Alleviates Airway Inflammation and Hyperreactivity in Ovalbumin-Sensitized Murine Model of Asthma.Evid Based Complement Alternat Med. 2015;2015:324265. doi: 10.1155/2015/324265. Epub 2015 Apr 20. Evid Based Complement Alternat Med. 2015. PMID: 26064160 Free PMC article.
-
Chemotaxis of bone marrow derived eosinophils in vivo: a novel method to explore receptor-dependent trafficking in the mouse.Eur J Immunol. 2013 Aug;43(8):2217-28. doi: 10.1002/eji.201343371. Epub 2013 Jun 14. Eur J Immunol. 2013. PMID: 23670593 Free PMC article.
-
Serum level of interleukin-13 receptor alpha 2 in infants with biliary atresia - is it of value?Clin Exp Hepatol. 2018 Jun;4(2):91-96. doi: 10.5114/ceh.2018.75958. Epub 2018 May 25. Clin Exp Hepatol. 2018. PMID: 29904725 Free PMC article.
References
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM (2000) Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161: 1720–1745. - PubMed
-
- Komiya A, Nagase H, Yamada H, Sekiya T, Yamaguchi M, et al. (2003) Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol 225: 91–100. - PubMed
-
- Ferland C, Guilbert M, Davoine F, Flamand N, Chakir J, et al. (2001) Eotaxin promotes eosinophil transmigration via the activation of the plasminogen-plasmin system. J Leukoc Biol 69: 772–778. - PubMed
-
- Pease JE, Williams TJ (2001) Eotaxin and asthma. Curr Opin Pharmacol 1: 248–253. - PubMed
-
- Banwell ME, Tolley NS, Williams TJ, Mitchell TJ (2002) Regulation of human eotaxin-3/CCL26 expression: modulation by cytokines and glucocorticoids. Cytokine 17: 317–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

