Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration
- PMID: 23022892
- PMCID: PMC3609049
- DOI: 10.4161/pri.22380
Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration
Abstract
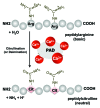
The post-translational citrullination (deimination) process is mediated by peptidylarginine deiminases (PADs), which convert peptidylarginine into peptidylcitrulline in the presence of high calcium concentrations. Over the past decade, PADs and protein citrullination have been commonly implicated as abnormal pathological features in neurodegeneration and inflammatory responses associated with diseases such as multiple sclerosis, Alzheimer disease and rheumatoid arthritis. Based on this evidence, we investigated the roles of PADs and citrullination in the pathogenesis of prion diseases. Prion diseases (also known as transmissible spongiform encephalopathies) are fatal neurodegenerative diseases that are pathologically well characterized as the accumulation of disease-associated misfolded prion proteins, spongiform changes, glial cell activation and neuronal loss. We previously demonstrated that the upregulation of PAD2, mainly found in reactive astrocytes of infected brains, leads to excessive citrullination, which is correlated with disease progression. Further, we demonstrated that various cytoskeletal and energy metabolism-associated proteins are particularly vulnerable to citrullination. Our recent in vivo and in vitro studies elicited altered functions of enolase as the result of citrullination; these altered functions included reduced enzyme activity, increased protease sensitivity and enhanced plasminogen-binding affinity. These findings suggest that PAD2 and citrullinated proteins may play a key role in the brain pathology of prion diseases. By extension, we believe that abnormal increases in protein citrullination may be strong evidence of neurodegeneration.
Keywords: citrullination; enolase; neurodegeneration; peptidylarginine deiminase; prion.
Figures
Similar articles
-
Accumulation of citrullinated proteins by up-regulated peptidylarginine deiminase 2 in brains of scrapie-infected mice: a possible role in pathogenesis.Am J Pathol. 2008 Oct;173(4):1129-42. doi: 10.2353/ajpath.2008.080388. Epub 2008 Sep 11. Am J Pathol. 2008. PMID: 18787103 Free PMC article.
-
Involvement of peptidylarginine deiminase-mediated post-translational citrullination in pathogenesis of sporadic Creutzfeldt-Jakob disease.Acta Neuropathol. 2010 Feb;119(2):199-210. doi: 10.1007/s00401-009-0625-x. Epub 2009 Dec 16. Acta Neuropathol. 2010. PMID: 20013286
-
Peptidylarginine deiminase modulates the physiological roles of enolase via citrullination: links between altered multifunction of enolase and neurodegenerative diseases.Biochem J. 2012 Jul 15;445(2):183-92. doi: 10.1042/BJ20120025. Biochem J. 2012. PMID: 22551201
-
Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination.Mol Cell Proteomics. 2014 Feb;13(2):388-96. doi: 10.1074/mcp.R113.033746. Epub 2013 Dec 2. Mol Cell Proteomics. 2014. PMID: 24298040 Free PMC article. Review.
-
Role of Peptidylarginine Deiminase 4 in Central Nervous System Diseases.Mol Neurobiol. 2023 Nov;60(11):6748-6756. doi: 10.1007/s12035-023-03489-3. Epub 2023 Jul 22. Mol Neurobiol. 2023. PMID: 37480499 Review.
Cited by
-
Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis.Biochim Biophys Acta. 2013 Oct;1829(10):1126-35. doi: 10.1016/j.bbagrm.2013.07.003. Epub 2013 Jul 13. Biochim Biophys Acta. 2013. PMID: 23860259 Free PMC article. Review.
-
The Peptidylarginine Deiminase Inhibitor Cl-Amidine Suppresses Inducible Nitric Oxide Synthase Expression in Dendritic Cells.Int J Mol Sci. 2017 Oct 27;18(11):2258. doi: 10.3390/ijms18112258. Int J Mol Sci. 2017. PMID: 29077055 Free PMC article.
-
Accumulation of citrullinated glial fibrillary acidic protein in a mouse model of bile duct ligation-induced hepatic fibrosis.PLoS One. 2018 Aug 2;13(8):e0201744. doi: 10.1371/journal.pone.0201744. eCollection 2018. PLoS One. 2018. PMID: 30071078 Free PMC article.
-
The Extracellular Vesicle Citrullinome and Signature in a Piglet Model of Neonatal Seizures.Int J Mol Sci. 2023 Jul 16;24(14):11529. doi: 10.3390/ijms241411529. Int J Mol Sci. 2023. PMID: 37511288 Free PMC article.
-
Protein citrullination marks myelin protein aggregation and disease progression in mouse ALS models.Acta Neuropathol Commun. 2022 Sep 8;10(1):135. doi: 10.1186/s40478-022-01433-5. Acta Neuropathol Commun. 2022. PMID: 36076282 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous