Iron-inducible nuclear translocation of a Myb3 transcription factor in the protozoan parasite Trichomonas vaginalis
- PMID: 23042127
- PMCID: PMC3536277
- DOI: 10.1128/EC.00190-12
Iron-inducible nuclear translocation of a Myb3 transcription factor in the protozoan parasite Trichomonas vaginalis
Abstract
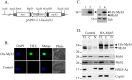
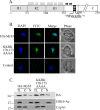
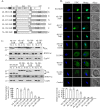
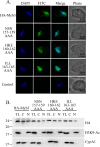
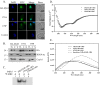
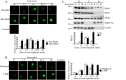
In Trichomonas vaginalis, a novel nuclear localization signal spanning the folded R2R3 DNA-binding domain of a Myb2 protein was previously identified. To study whether a similar signal is used for nuclear translocation by other Myb proteins, nuclear translocation of Myb3 was examined in this report. When overexpressed, hemagglutinin-tagged Myb3 was localized to nuclei of transfected cells, with a cellular distribution similar to that of endogenous Myb3. Fusion to a bacterial tetracycline repressor, R2R3, of Myb3 that spans amino acids (aa) 48 to 156 was insufficient for nuclear translocation of the fusion protein, unless its C terminus was extended to aa 167. The conserved isoleucine in helix 2 of R2R3, which is important for Myb2's structural integrity in maintaining DNA-binding activity and nuclear translocation, was also vital for the former activity of Myb3, but less crucial for the latter. Sequential nuclear influx and efflux of Myb3, which require further extension of the nuclear localization signal to aa 180, were immediately induced after iron repletion. Sequence elements that regulate nuclear translocation with cytoplasmic retention, nuclear influx, and nuclear efflux were identified within the C-terminal tail. These results suggest that the R2R3 DNA-binding domain also serves as a common module for the nuclear translocation of both Myb2 and Myb3, but there are intrinsic differences between the two nuclear localization signals.
Figures

Similar articles
-
A highly organized structure mediating nuclear localization of a Myb2 transcription factor in the protozoan parasite Trichomonas vaginalis.Eukaryot Cell. 2011 Dec;10(12):1607-17. doi: 10.1128/EC.05177-11. Epub 2011 Oct 21. Eukaryot Cell. 2011. PMID: 22021237 Free PMC article.
-
Signal transduction triggered by iron to induce the nuclear importation of a Myb3 transcription factor in the parasitic protozoan Trichomonas vaginalis.J Biol Chem. 2014 Oct 17;289(42):29334-49. doi: 10.1074/jbc.M114.599498. Epub 2014 Sep 2. J Biol Chem. 2014. PMID: 25183012 Free PMC article.
-
Transcriptional regulation of an iron-inducible gene by differential and alternate promoter entries of multiple Myb proteins in the protozoan parasite Trichomonas vaginalis.Eukaryot Cell. 2009 Mar;8(3):362-72. doi: 10.1128/EC.00317-08. Epub 2009 Jan 16. Eukaryot Cell. 2009. PMID: 19151329 Free PMC article.
-
RNA-Binding Proteins in Trichomonas vaginalis: Atypical Multifunctional Proteins.Biomolecules. 2015 Nov 26;5(4):3354-95. doi: 10.3390/biom5043354. Biomolecules. 2015. PMID: 26703754 Free PMC article. Review.
-
Trichomonas vaginalis adhesin proteins display molecular mimicry to metabolic enzymes.Adv Exp Med Biol. 1996;408:207-23. doi: 10.1007/978-1-4613-0415-9_25. Adv Exp Med Biol. 1996. PMID: 8895795 Review. No abstract available.
Cited by
-
Structural basis of interaction between dimeric cyclophilin 1 and Myb1 transcription factor in Trichomonas vaginalis.Sci Rep. 2018 Apr 3;8(1):5410. doi: 10.1038/s41598-018-23821-5. Sci Rep. 2018. PMID: 29615721 Free PMC article.
-
Morphologic study of the effect of iron on pseudocyst formation in Trichomonas vaginalis and its interaction with human epithelial cells.Mem Inst Oswaldo Cruz. 2017 Oct;112(10):664-673. doi: 10.1590/0074-02760170032. Mem Inst Oswaldo Cruz. 2017. PMID: 28953994 Free PMC article.
-
Regulation of nuclear translocation of the Myb1 transcription factor by TvCyclophilin 1 in the protozoan parasite Trichomonas vaginalis.J Biol Chem. 2014 Jul 4;289(27):19120-36. doi: 10.1074/jbc.M114.549410. Epub 2014 May 15. J Biol Chem. 2014. PMID: 24831011 Free PMC article.
-
The Non-Canonical Iron-Responsive Element of IRE-tvcp12 Hairpin Structure at the 3'-UTR of Trichomonas vaginalis TvCP12 mRNA That Binds TvHSP70 and TvACTN-3 Can Regulate mRNA Stability and Amount of Protein.Pathogens. 2023 Apr 12;12(4):586. doi: 10.3390/pathogens12040586. Pathogens. 2023. PMID: 37111472 Free PMC article.
-
The cell survival pathways of the primordial RNA-DNA complex remain conserved in the extant genomes and may function as proto-oncogenes.Eur J Microbiol Immunol (Bp). 2015 Mar;5(1):25-43. doi: 10.1556/EUJMI-D-14-00034. Epub 2015 Mar 26. Eur J Microbiol Immunol (Bp). 2015. PMID: 25883792 Free PMC article. Review.
References
-
- Alderete JF, Provenzano D, Lehker HW. 1995. Iron mediates Trichomonas vaginalis resistance to complement lysis. Microb. Pathog. 19:93–103 - PubMed
-
- Alderete JF, et al. 1995. Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 17:69–83 - PubMed
-
- Alderete JF, Milsap KW, Lehker MW, Benchimol M. 2001. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell Microbiol. 3:359–370 - PubMed
-
- Alderete JF, Nguyen J, Mundodi V, Lehker HW. 2004. Heme-iron increases level of AP65-mediated adherence by Trichomonas vaginalis. Microb. Pathog. 36:263–271 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

