Triflumizole is an obesogen in mice that acts through peroxisome proliferator activated receptor gamma (PPARγ)
- PMID: 23086663
- PMCID: PMC3548286
- DOI: 10.1289/ehp.1205383
Triflumizole is an obesogen in mice that acts through peroxisome proliferator activated receptor gamma (PPARγ)
Abstract
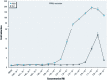
Background: Triflumizole (TFZ) is an imidazole fungicide used on many food and ornamental crops. TFZ is not thought to be particularly toxic or carcinogenic, but little is known about its effect on development. TFZ is identified as a peroxisome proliferator activated receptor gamma (PPARγ) activator in ToxCast. Because PPARγ is a master regulator of adipogenesis, we hypothesized that TFZ would activate PPARγ, thereby inducing adipogenesis and weight gain in vivo.
Objectives: We sought to test the ability of TFZ to activate PPARγ and promote adipogenesis in vitro and in vivo.
Methods: We used transient transfection to test the ability of TFZ to activate PPARγ, and we used 3T3-L1 preadipocytes and human multipotent mesenchymal stromal stem cells (MSCs) to study the adipogenic capacity of TFZ in culture. We treated pregnant mice with three doses of TFZ and evaluated the effects on body weight, adipose depot weight, and MSC programming in the prenatally exposed offspring.
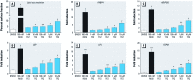
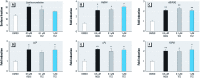
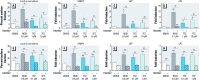
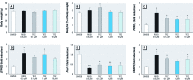
Discussion: TFZ induced adipogenesis in MSCs and in mouse 3T3-L1 preadipocytes. Prenatal exposure to levels of TFZ at approximately 400-fold below the reported no observed adverse effect level increased adipose depot weight. All doses of TFZ tested increased adipogenic gene expression in MSCs while inhibiting expression of osteogenic genes.
Conclusions: TFZ acts through a PPARγ-dependent mechanism to induce adipogenic differentiation in MSCs and preadipocytes at low nanomolar concentrations. Prenatal TFZ exposure increases adipose depot weight and diverts MSC fate toward the adipocyte lineage; therefore, we conclude that TFZ is an obesogen in vivo.
Conflict of interest statement
B.B. is a named inventor on U.S. patents 5,861,274, 6,200,802, 6,815,168, and 7,250,273 related to PPARγ. The other authors declare they have no actual or potential competing financial interests.
Figures
Comment in
-
Potential obesogen identified: fungicide triflumizole is associated with increased adipogenesis in mice.Environ Health Perspect. 2012 Dec;120(12):A474. doi: 10.1289/ehp.120-a474a. Environ Health Perspect. 2012. PMID: 23211365 Free PMC article. No abstract available.
Similar articles
-
The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes.J Steroid Biochem Mol Biol. 2011 Oct;127(1-2):9-15. doi: 10.1016/j.jsbmb.2011.03.012. Epub 2011 Mar 21. J Steroid Biochem Mol Biol. 2011. PMID: 21397693 Free PMC article.
-
Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism.Environ Health Perspect. 2012 Jul;120(7):984-9. doi: 10.1289/ehp.1205063. Epub 2012 May 25. Environ Health Perspect. 2012. PMID: 22763116 Free PMC article.
-
PPARγ agonist through the terminal differentiation phase is essential for adipogenic differentiation of fetal ovine preadipocytes.Cell Mol Biol Lett. 2017 Mar 23;22:6. doi: 10.1186/s11658-017-0037-1. eCollection 2017. Cell Mol Biol Lett. 2017. PMID: 28536637 Free PMC article.
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
-
Current methods of adipogenic differentiation of mesenchymal stem cells.Stem Cells Dev. 2011 Oct;20(10):1793-804. doi: 10.1089/scd.2011.0040. Epub 2011 Jun 20. Stem Cells Dev. 2011. PMID: 21526925 Free PMC article. Review.
Cited by
-
Editor's Highlight: Screening ToxCast Prioritized Chemicals for PPARG Function in a Human Adipose-Derived Stem Cell Model of Adipogenesis.Toxicol Sci. 2017 Jan;155(1):85-100. doi: 10.1093/toxsci/kfw186. Epub 2016 Sep 23. Toxicol Sci. 2017. PMID: 27664422 Free PMC article.
-
Worker illness related to newly marketed pesticides--Douglas County, Washington, 2014.MMWR Morb Mortal Wkly Rep. 2015 Jan 23;64(2):42-4. MMWR Morb Mortal Wkly Rep. 2015. PMID: 25611169 Free PMC article.
-
Comment on "On the Utility of ToxCast™ and ToxPi as Methods for Identifying New Obesogens".Environ Health Perspect. 2017 Jan 1;125(1):A8-A11. doi: 10.1289/EHP881. Environ Health Perspect. 2017. PMID: 28055944 Free PMC article. No abstract available.
-
Generalized Concentration Addition Modeling Predicts Mixture Effects of Environmental PPARγ Agonists.Toxicol Sci. 2016 Sep;153(1):18-27. doi: 10.1093/toxsci/kfw100. Epub 2016 Jun 2. Toxicol Sci. 2016. PMID: 27255385 Free PMC article.
-
Triflumizole Induces Developmental Toxicity, Liver Damage, Oxidative Stress, Heat Shock Response, Inflammation, and Lipid Synthesis in Zebrafish.Toxics. 2022 Nov 17;10(11):698. doi: 10.3390/toxics10110698. Toxics. 2022. PMID: 36422906 Free PMC article.
References
-
- Burris TP, Pelton PD, Zhou L, Osborne MC, Cryan E, Demarest KT. A novel method for analysis of nuclear receptor function at natural promoters: peroxisome proliferator-activated receptor γ agonist actions on aP2 gene expression detected using branched DNA messenger RNA quantitation. Mol Endocrinol. 1999;13(3):410–417. - PubMed
-
- Chamorro-Garcia R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, et al. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator–activated receptor gamma-independent mechanism. Environ Health Perspect. 2012;120:984–989. - PMC - PubMed
-
- Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 2007;95(1):5–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

