Fenretinide inhibited de novo ceramide synthesis and proinflammatory cytokines induced by Aggregatibacter actinomycetemcomitans
- PMID: 23139430
- PMCID: PMC3520524
- DOI: 10.1194/jlr.M031427
Fenretinide inhibited de novo ceramide synthesis and proinflammatory cytokines induced by Aggregatibacter actinomycetemcomitans
Abstract
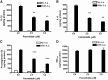
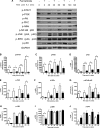
Ceramides play an essential role in modulating immune signaling pathways and proinflammatory cytokine production in response to infectious pathogens, stress stimuli, or chemotherapeutic drugs. In this study, we demonstrated that Aggregatibacter actinomycetemcomitans, the pathogen for aggressive periodontitis, induced de novo synthesis of ceramide in Raw 264.7 cells. In addition, we identified that fenretinide, a synthetic retinoid, suppressed the de novo synthesis of ceramide induced by A. actinomycetemcomitans. Moreover, fenretinide attenuated interleukin (IL)-1β, IL-6, and cyclooxygenase-2 mRNA expression induced by A. actinomycetemcomitans. Fenretinide also decreased IL-1β, IL-6, and prostaglandin E2 proinflammatory cytokine levels in Raw 264.7 cells induced by A. actinomycetemcomitans. However, fenretinide had no significant effects on tumor necrosis factor alpha mRNA or protein levels. Furthermore, we showed that fenretinide inhibited the janus kinase-signal transducer and activator of transcription, phosphatidylinositol 3-kinase-Akt, protein kinase C, and nuclear factor-kappaB signaling pathways, whereas fenretinide up-regulated the mitogen-activated protein kinase signaling pathways after bacterial stimulation. This study emphasizes the de novo ceramide synthesis pathway in response to bacterial stimulation and demonstrates the anti-inflammatory role of fenretinide in the bacteria-induced immune response.
Figures

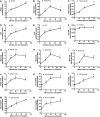
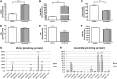
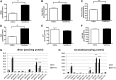

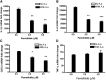
Similar articles
-
FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans.Lipids Health Dis. 2015 Jul 4;14:66. doi: 10.1186/s12944-015-0057-7. Lipids Health Dis. 2015. PMID: 26138336 Free PMC article.
-
Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway.J Biol Chem. 2011 Aug 12;286(32):28556-66. doi: 10.1074/jbc.M111.256180. Epub 2011 Jun 13. J Biol Chem. 2011. PMID: 21669872 Free PMC article.
-
4-methoxycinnamyl p-coumarate isolated from Etlingera pavieana rhizomes inhibits inflammatory response via suppression of NF-κB, Akt and AP-1 signaling in LPS-stimulated RAW 264.7 macrophages.Phytomedicine. 2019 Feb 15;54:89-97. doi: 10.1016/j.phymed.2018.09.193. Epub 2018 Sep 18. Phytomedicine. 2019. PMID: 30668386
-
Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells.Immunobiology. 2013 Dec;218(12):1452-67. doi: 10.1016/j.imbio.2013.04.019. Epub 2013 May 9. Immunobiology. 2013. PMID: 23735482
-
Vibrio harveyi infections induce production of proinflammatory cytokines in murine peritoneal macrophages via activation of p38 MAPK and NF-κB pathways, but reversed by PI3K/AKT pathways.Dev Comp Immunol. 2022 Feb;127:104292. doi: 10.1016/j.dci.2021.104292. Epub 2021 Oct 14. Dev Comp Immunol. 2022. PMID: 34656643 Review.
Cited by
-
Phosphoglycerol dihydroceramide, a distinctive ceramide produced by Porphyromonas gingivalis, promotes RANKL-induced osteoclastogenesis by acting on non-muscle myosin II-A (Myh9), an osteoclast cell fusion regulatory factor.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 May;1862(5):452-462. doi: 10.1016/j.bbalip.2017.01.008. Epub 2017 Jan 31. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28153611 Free PMC article.
-
The Sphingolipid-Signaling Pathway as a Modulator of Infection by SARS-CoV-2.Curr Issues Mol Biol. 2023 Sep 28;45(10):7956-7973. doi: 10.3390/cimb45100503. Curr Issues Mol Biol. 2023. PMID: 37886946 Free PMC article. Review.
-
Pulmonary Delivery of Fenretinide: A Possible Adjuvant Treatment In COVID-19.Int J Mol Sci. 2020 May 27;21(11):3812. doi: 10.3390/ijms21113812. Int J Mol Sci. 2020. PMID: 32471278 Free PMC article. Review.
-
Ceramide Imbalance and Impaired TLR4-Mediated Autophagy in BMDM of an ORMDL3-Overexpressing Mouse Model.Int J Mol Sci. 2019 Mar 20;20(6):1391. doi: 10.3390/ijms20061391. Int J Mol Sci. 2019. PMID: 30897694 Free PMC article.
-
FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans.Lipids Health Dis. 2015 Jul 4;14:66. doi: 10.1186/s12944-015-0057-7. Lipids Health Dis. 2015. PMID: 26138336 Free PMC article.
References
-
- Hannun Y. A., Obeid L. M. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150 - PubMed
-
- Grassme H., Riethmuller J., Gulbins E. 2007. Biological aspects of ceramide-enriched membrane domains. Prog. Lipid Res. 46: 161–170 - PubMed
-
- Schenck M., Carpinteiro A., Grassme H., Lang F., Gulbins E. 2007. Ceramide: physiological and pathophysiological aspects. Arch. Biochem. Biophys. 462: 171–175 - PubMed
-
- Zanardi S., Serrano D., Argusti A., Barile M., Puntoni M., Decensi A. 2006. Clinical trials with retinoids for breast cancer chemoprevention. Endocr. Relat. Cancer. 13: 51–68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

