Isolation and characterization of human dental pulp derived stem cells by using media containing low human serum percentage as clinical grade substitutes for bovine serum
- PMID: 23155430
- PMCID: PMC3498354
- DOI: 10.1371/journal.pone.0048945
Isolation and characterization of human dental pulp derived stem cells by using media containing low human serum percentage as clinical grade substitutes for bovine serum
Abstract
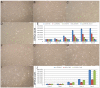
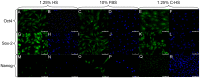
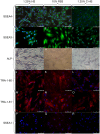
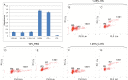
Adult stem cells have been proposed as an alternative to embryonic stem cells to study multilineage differentiation in vitro and to use in therapy. Current culture media for isolation and expansion of adult stem cells require the use of large amounts of animal sera, but animal-derived culture reagents give rise to some questions due to the real possibility of infections and severe immune reactions. For these reasons a clinical grade substitute to animal sera is needed. We tested the isolation, proliferation, morphology, stemness related marker expression, and osteoblastic differentiation potential of Dental Pulp Stem Cells (DPSC) in a chemically defined medium containing a low percentage of human serum, 1.25%, in comparison to a medium containing 10% Fetal Bovine Serum (FBS). DPSCs cultured in presence of our isolation/proliferation medium added with low HS percentage were obtained without immune-selection methods and showed high uniformity in the expression of stem cell markers, proliferated at higher rate, and demonstrated comparable osteoblastic potential with respect to DPSCs cultured in 10% FBS. In this study we demonstrated that a chemically defined medium added with low HS percentage, derived from autologous and heterologous sources, could be a valid substitute to FBS-containing media and should be helpful for adult stem cells clinical application.
Conflict of interest statement
Figures

Similar articles
-
Dental pulp stem cell (DPSC) isolation, characterization, and differentiation.Methods Mol Biol. 2014;1210:91-115. doi: 10.1007/978-1-4939-1435-7_8. Methods Mol Biol. 2014. PMID: 25173163
-
Tooth storage, dental pulp stem cell isolation, and clinical scale expansion without animal serum.J Endod. 2014 May;40(5):652-7. doi: 10.1016/j.joen.2014.01.005. Epub 2014 Mar 6. J Endod. 2014. PMID: 24767559
-
Establishment of xenogeneic serum-free culture methods for handling human dental pulp stem cells using clinically oriented in-vitro and in-vivo conditions.Stem Cell Res Ther. 2018 Feb 3;9(1):25. doi: 10.1186/s13287-017-0761-5. Stem Cell Res Ther. 2018. PMID: 29394956 Free PMC article.
-
The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars.Biomaterials. 2012 Jul;33(20):5023-35. doi: 10.1016/j.biomaterials.2012.03.057. Epub 2012 Apr 18. Biomaterials. 2012. PMID: 22516606
-
In Vitro Culturing of Adult Stem Cells: The Importance of Serum and Atmospheric Oxygen.Adv Exp Med Biol. 2022;1376:101-118. doi: 10.1007/5584_2021_656. Adv Exp Med Biol. 2022. PMID: 34426961 Review.
Cited by
-
Serum and supplement optimization for EU GMP-compliance in cardiospheres cell culture.J Cell Mol Med. 2014 Apr;18(4):624-34. doi: 10.1111/jcmm.12210. Epub 2014 Jan 20. J Cell Mol Med. 2014. PMID: 24444305 Free PMC article.
-
Human dental pulp stem cells cultured in serum-free supplemented medium.Front Physiol. 2013 Dec 11;4:357. doi: 10.3389/fphys.2013.00357. eCollection 2013. Front Physiol. 2013. PMID: 24376422 Free PMC article.
-
Minor salivary gland stem cells: a comparative study of the biological properties under clinical-grade culture conditions.Cell Tissue Res. 2023 Aug;393(2):321-342. doi: 10.1007/s00441-023-03789-z. Epub 2023 May 30. Cell Tissue Res. 2023. PMID: 37249709 Free PMC article.
-
Good manufacturing practice production of human corneal limbus-derived stromal stem cells and in vitro quality screening for therapeutic inhibition of corneal scarring.Stem Cell Res Ther. 2024 Jan 8;15(1):11. doi: 10.1186/s13287-023-03626-8. Stem Cell Res Ther. 2024. PMID: 38185673 Free PMC article.
-
Dental Pulp Stem Cells: Current Advances in Isolation, Expansion and Preservation.Tissue Eng Regen Med. 2017 Mar 14;14(4):333-347. doi: 10.1007/s13770-017-0036-3. eCollection 2017 Aug. Tissue Eng Regen Med. 2017. PMID: 30603490 Free PMC article. Review.
References
-
- Ulloa-Montoya F, Verfaillie CM, Hu WS (2005) Culture systems for pluripotent stem cells. J Biosci Bioeng 100: 12–27. - PubMed
-
- Van der Valka J, Mellorb D, Brandsc R (2004) The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxic in Vitro 18: 1–12. - PubMed
-
- Eloit M (1999) Risks of virus transmission associated with animal sera or substitutes and methods of control. Dev Biol Stand 99: 9–16. - PubMed
-
- Shah G (1999) Why do we still use serum in the production of biopharmaceuticals? Dev Biol Stand 99: 17–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

