The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55
- PMID: 23161546
- PMCID: PMC3531739
- DOI: 10.1074/jbc.M112.364109
The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55
Abstract
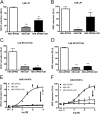
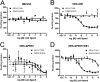
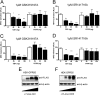
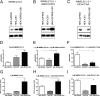
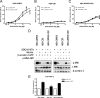
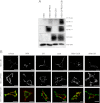
The G protein-coupled receptor (GPCR) 55 (GPR55) and the cannabinoid receptor 1 (CB1R) are co-expressed in many tissues, predominantly in the central nervous system. Seven transmembrane spanning (7TM) receptors/GPCRs can form homo- and heteromers and initiate distinct signaling pathways. Recently, several synthetic CB1 receptor inverse agonists/antagonists, such as SR141716A, AM251, and AM281, were reported to activate GPR55. Of these, SR141716A was marketed as a promising anti-obesity drug, but was withdrawn from the market because of severe side effects. Here, we tested whether GPR55 and CB1 receptors are capable of (i) forming heteromers and (ii) whether such heteromers could exhibit novel signaling patterns. We show that GPR55 and CB1 receptors alter each others signaling properties in human embryonic kidney (HEK293) cells. We demonstrate that the co-expression of FLAG-CB1 receptors in cells stably expressing HA-GPR55 specifically inhibits GPR55-mediated transcription factor activation, such as nuclear factor of activated T-cells and serum response element, as well as extracellular signal-regulated kinases (ERK1/2) activation. GPR55 and CB1 receptors can form heteromers, but the internalization of both receptors is not affected. In addition, we observe that the presence of GPR55 enhances CB1R-mediated ERK1/2 and nuclear factor of activated T-cell activation. Our data provide the first evidence that GPR55 can form heteromers with another 7TM/GPCR and that this interaction with the CB1 receptor has functional consequences in vitro. The GPR55-CB1R heteromer may play an important physiological and/or pathophysiological role in tissues endogenously co-expressing both receptors.
Figures



Similar articles
-
A selective antagonist reveals a potential role of G protein-coupled receptor 55 in platelet and endothelial cell function.J Pharmacol Exp Ther. 2013 Jul;346(1):54-66. doi: 10.1124/jpet.113.204180. Epub 2013 May 2. J Pharmacol Exp Ther. 2013. PMID: 23639801
-
CB1 and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum.Exp Neurol. 2014 Nov;261:44-52. doi: 10.1016/j.expneurol.2014.06.017. Epub 2014 Jun 23. Exp Neurol. 2014. PMID: 24967683
-
Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling.Br J Pharmacol. 2014 Dec;171(23):5387-406. doi: 10.1111/bph.12850. Epub 2014 Sep 5. Br J Pharmacol. 2014. PMID: 25048571 Free PMC article.
-
GPR55, a lysophosphatidylinositol receptor with cannabinoid sensitivity?Curr Top Med Chem. 2010;10(8):799-813. doi: 10.2174/156802610791164229. Curr Top Med Chem. 2010. PMID: 20370712 Review.
-
Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55.Mol Endocrinol. 2011 Nov;25(11):1835-48. doi: 10.1210/me.2011-1197. Epub 2011 Sep 29. Mol Endocrinol. 2011. PMID: 21964594 Free PMC article. Review.
Cited by
-
The Role of Cannabidiol in Liver Disease: A Systemic Review.Int J Mol Sci. 2024 Feb 17;25(4):2370. doi: 10.3390/ijms25042370. Int J Mol Sci. 2024. PMID: 38397045 Free PMC article. Review.
-
The Endocannabinoid System as a _target in Cancer Diseases: Are We There Yet?Front Pharmacol. 2019 Apr 5;10:339. doi: 10.3389/fphar.2019.00339. eCollection 2019. Front Pharmacol. 2019. PMID: 31024307 Free PMC article. Review.
-
Epidemiological overview of multidimensional chromosomal and genome toxicity of cannabis exposure in congenital anomalies and cancer development.Sci Rep. 2021 Jul 6;11(1):13892. doi: 10.1038/s41598-021-93411-5. Sci Rep. 2021. PMID: 34230557 Free PMC article.
-
GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis.Br J Pharmacol. 2016 Jan;173(1):142-54. doi: 10.1111/bph.13345. Epub 2015 Nov 25. Br J Pharmacol. 2016. PMID: 26436760 Free PMC article.
-
What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity?Nutrients. 2021 Jan 26;13(2):373. doi: 10.3390/nu13020373. Nutrients. 2021. PMID: 33530406 Free PMC article. Review.
References
-
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 - PubMed
-
- Munro S., Thomas K. L., Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 - PubMed
-
- Pertwee R. G., Howlett A. C., Abood M. E., Alexander S. P., Di Marzo V., Elphick M. R., Greasley P. J., Hansen H. S., Kunos G., Mackie K., Mechoulam R., Ross R. A. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands. Beyond CB1 and CB2. Pharmacol. Rev. 62, 588–631 - PMC - PubMed
-
- Tsou K., Brown S., Sañudo-Peña M. C., Mackie K., Walker J. M. (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

